An alternative in vivo model to evaluate pluripotency of patient-specific iPSCs
Main Article Content
Abstract
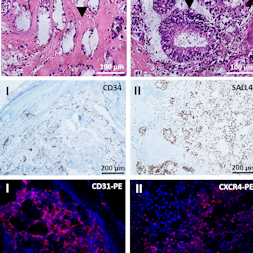
The generation of autologous human induced pluripotent stem cells (hiPSCs) from a patient’s somatic cells and the subsequent differentiation of these cells into desired cell types offer innovative treatment options for tissue regeneration. The hiPSCs obtained are usually implanted in immunodeficient mice, and teratoma formation is analyzed after 4 to 6 weeks to assess the cells’ pluripotency. In this study, an alternative in vivo model based on chicken egg chorioallantoic membrane (CAM) was established to analyze the pluripotency of newly created hiPSCs. 0.5, 1, 2, 4 x 106 hiPSCs generated from urine-derived renal epithelial cells were seeded on CAM and incubated for 9 days. Teratoma formation was detected in 70% of eggs inoculated with 2 x 106 hiPSCs and in 100% of eggs inoculated with 4 x 106 hiPSCs. All teratomas exhibited vascular structures. The robustness of the CAM model was confirmed using two additional hiPSC lines derived from human fibroblasts (NuFFs) or jaw periosteal cells. The presence of all three germ layers within the teratomas was successfully verified by histochemical and immunofluorescence staining and gene expression analysis of germ layer-specific markers. Urine-derived renal epithelial cells were used as negative control and showed no teratoma formation. The CAM-based in vivo model provides an optimal in vivo test environment for the pluripotency evaluation of newly generated hiPSC lines. This simple, fast, inexpensive and reproducible method reduces the suffering of animals and thus implements the principles of the 3Rs (replacement, reduction, and refinement).
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Aisenbrey, E. A. and Murphy, W. L. (2020). Synthetic alternatives to matrigel. Nat Rev Mater 5, 539-551. doi:10.1038/s41578-020-0199-8
Aldahmash, A., Atteya, M., Elsafadi, M. et al. (2013). Teratoma formation in immunocompetent mice after syngeneic and allogeneic implantation of germline capable mouse embryonic stem cells. Asian Pac J Cancer Prev 14, 5705-5711. doi:10.7314/apjcp.2013.14.10.5705
Buhr, C. R., Wiesmann, N., Tanner, R. C. et al. (2020). The chorioallantoic membrane assay in nanotoxicological research – An alternative for in vivo experimentation. Nanomaterials (Basel) 10, 2328. doi:10.3390/nano10122328
Buta, C., David, R., Dressel, R. et al. (2013). Reconsidering pluripotency tests: Do we still need teratoma assays? Stem Cell Res 11, 552-562. doi:10.1016/j.scr.2013.03.001
Cao, F., Lin, S., Xie, X. et al. (2006). In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 113, 1005-1014. doi:10.1161/circulationaha.105.588954
Cao, F., van der Bogt, K. E., Sadrzadeh, A. et al. (2007). Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev 16, 883-891. doi:10.1089/scd.2007.0160
Cieślar-Pobuda, A., Knoflach, V., Ringh, M. V. et al. (2017). Transdifferentiation and reprogramming: Overview of the processes, their similarities and differences. Biochim Biophys Acta Mol Cell Res 1864, 1359-1369. doi:10.1016/j.bbamcr.2017.04.017
DeBord, L. C., Pathak, R. R., Villaneuva, M. et al. (2018). The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am J Cancer Res 8, 1642-1660.
Deryugina, E. I. and Quigley, J. P. (2008). Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol 130, 1119-1130. doi:10.1007/s00418-008-0536-2
Dexter, D. L., Lee, E. S., DeFusco, D. J. et al. (1983). Selection of metastatic variants from heterogeneous tumor cell lines using the chicken chorioallantoic membrane and nude mouse. Cancer Res 43, 1733-1740.
Dohle, D. S., Pasa, S. D., Gustmann, S. et al. (2009). Chick ex ovo culture and ex ovo CAM assay: How it really works. J Vis Exp, e1620. doi:10.3791/1620
Durupt, F., Koppers-Lalic, D., Balme, B. et al. (2012). The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther 19, 58-68. doi:10.1038/cgt.2011.68
Gilleron, L., Coecke, S., Sysmans, M. et al. (1996). Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. Toxicol In Vitro 10, 431-446. doi:10.1016/0887-2333(96)00021-5
Hentze, H., Soong, P. L., Wang, S. T. et al. (2009). Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res 2, 198-210. doi:10.1016/j.scr.2009.02.002
Kaji, K., Norrby, K., Paca, A. et al. (2009). Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458, 771-775. doi:10.1038/nature07864
Kibbe, W. A. (2007). OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res 35, W43-46. doi:10.1093/nar/gkm234
Kunz, P., Schenker, A., Sahr, H. et al. (2019). Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS One 14, e0215312. doi:10.1371/journal.pone.0215312
Kunzi-Rapp, K., Genze, F., Kufer, R. et al. (2001). Chorioallantoic membrane assay: Vascularized 3-dimensional cell culture system for human prostate cancer cells as an animal substitute model. J Urol 166, 1502-1507. doi:10.1016/s0022-5347(05)65820-x
Lee, A. S., Tang, C., Cao, F. et al. (2009). Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 8, 2608-2612. doi:10.4161/cc.8.16.9353
Li, M., Pathak, R. R., Lopez-Rivera, E. et al. (2015). The in ovo chick chorioallantoic membrane (CAM) assay as an efficient xenograft model of hepatocellular carcinoma. J Vis Exp, e52411. doi:10.3791/52411
McDonald, D., Wu, Y., Dailamy, A. et al. (2020). Defining the teratoma as a model for multi-lineage human development. Cell 183, 1402-1419 e1418. doi:10.1016/j.cell.2020.10.018
Moreno-Jimenez, I., Hulsart-Billstrom, G., Lanham, S. A. et al. (2016). The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: A refinement animal model for tissue engineering. Sci Rep 6, 32168. doi:10.1038/srep32168
Naik, M., Brahma, P. and Dixit, M. (2018). A cost-effective and efficient chick ex-ovo cam assay protocol to assess angiogenesis. Methods Protoc 1, 19. doi:10.3390/mps1020019
Nelakanti, R. V., Kooreman, N. G. and Wu, J. C. (2015). Teratoma formation: A tool for monitoring pluripotency in stem cell research. Curr Protoc Stem Cell Biol 32, 4A.8.1-17. doi:10.1002/9780470151808.sc04a08s32
Petrovova, E., Giretova, M., Kvasilova, A. et al. (2019). Preclinical alternative model for analysis of porous scaffold biocompatibility in bone tissue engineering. ALTEX 36, 121-130. doi:10.14573/altex.1807241
Rabinovich, P. M. and Weissman, S. M. (2013). Cell engineering with synthetic messenger RNA. Methods Mol Biol 969, 3-28. doi:10.1007/978-1-62703-260-5_1
Rovithi, M., Avan, A., Funel, N. et al. (2017). Development of bioluminescent chick chorioallantoic membrane (CAM) models for primary pancreatic cancer cells: A platform for drug testing. Sci Rep 7, 44686. doi:10.1038/srep44686
Steinle, H., Behring, A., Schlensak, C. et al. (2017). Concise review: Application of in vitro transcribed messenger RNA for cellular engineering and reprogramming: Progress and challenges. Stem Cells 35, 68-79. doi:10.1002/stem.2402
Steinle, H., Golombek, S., Behring, A. et al. (2018). Improving the angiogenic potential of EPCs via engineering with synthetic modified mRNAs. Mol Ther Nucleic Acids 13, 387-398. doi:10.1016/j.omtn.2018.09.005
Steinle, H., Weber, M., Behring, A. et al. (2019a). Generation of iPSCs by nonintegrative RNA-based reprogramming techniques: Benefits of self-replicating RNA versus synthetic mRNA. Stem Cells Int 2019, 7641767. doi:10.1155/2019/7641767
Steinle, H., Weber, M., Behring, A. et al. (2019b). Reprogramming of urine-derived renal epithelial cells into iPSCs using srRNA and consecutive differentiation into beating cardiomyocytes. Mol Ther Nucleic Acids 17, 907-921. doi:10.1016/j.omtn.2019.07.016
Takahashi, K. and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. doi:10.1016/j.cell.2006.07.024
Takahashi, K., Tanabe, K., Ohnuki, M. et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861-872. doi:10.1016/j.cell.2007.11.019
Warren, L., Manos, P. D., Ahfeldt, T. et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618-630. doi:10.1016/j.stem.2010.08.012
Winter, G., Koch, A. B. F., Loffler, J. et al. (2020). Multi-modal PET and MR imaging in the hen’s egg test-chorioallantoic membrane (HET-CAM) model for initial in vivo testing of target-specific radioligands. Cancers (Basel) 12, 1248. doi:10.3390/cancers12051248
Ye, J., Coulouris, G., Zaretskaya, I. et al. (2012). Primer-blast: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134. doi:10.1186/1471-2105-13-134
Ying, Y., Xingfen, Y., Wengai, Z. et al. (2010). Combined in vitro tests as an alternative to in vivo eye irritation tests. Altern Lab Anim 38, 303-314. doi:10.1177/026119291003800413
Yu, J., Hu, K., Smuga-Otto, K. et al. (2009). Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797-801. doi:10.1126/science.1172482
Zhang, W. Y., de Almeida, P. E. and Wu, J. C. (2008). Teratoma formation: A tool for monitoring pluripotency in stem cell research. In (ed.), StemBook. Cambridge (MA). doi:10.3824/stembook.1.53.1