Detection of low levels of genotoxic compounds in food contact materials using an alternative HPTLC-SOS-Umu-C assay
Main Article Content
Abstract
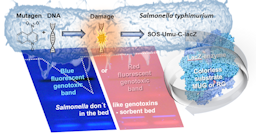
Food contact materials (FCMs) are perceived as major sources of chemical food contamination, bringing significant safety uncertainties into the food chain. Consequently, there has been an increasing demand to improve hazard and risk assessment of FCMs. High-performance thin-layer chromatography (HPTLC) coupled to a genotoxicity bioassay has been promoted as an alternative approach to assess food packaging migrates. To investigate the value of such a testing approach, a sensitive planar SOS-Umu-C assay has been developed using the Salmonella strain. The new conditions established based on HPTLC were verified by comparison with microtiter plate assays, the Ames and Salmonella-SOS-Umu-C assays. The lowest effective concentration of the genotoxin 4-nitroquinoline-1-oxide (0.53 nM; 20 pg/band) in the SOS-Umu-C assay was 176 times lower than in the microtiter plate counterpart. This was achieved by the developed chromatographic setup, including a fluorogenic instead of chromogenic substrate. As proof-of-principle, FCM extracts and migrates from differently coated tin cans were analyzed. The performance data highlighted reliable dose-response curves, good mean reproducibility, no quenching or other matrix effects, no solvent exposure limitations, and no need for a solid phase extraction or concentration step due to high sensitivity in the picomolar range. Although further performance developments of the assay are still needed, the developed planar assay was successfully proven to work quantitatively in the food packaging field.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Baumann, U., Brunner, C., Pletscher, E. et al. (2003). Biologische Detektionsverfahren in der Dünnschichtchromatographie (Biological detection procedures using thin-layer chromatography). UWSF – Z Umweltchem Ökotox 15, 163-167. doi:10.1065/uwsf2001.12.080
Bjorseth, A., Eidsa, G., Gether, J. et al. (1982). Detection of mutagens in complex samples by the Salmonella assay applied directly on thin-layer chromatography plates. Science 215, 87-89. doi:10.1126/science.7031897
Bopp, S. K., Kienzler, A., Richarz, A.-N. et al. (2019). Regulatory assessment and risk management of chemical mixtures: Challenges and ways forward. Crit Rev Toxicol 49, 174-189. doi:10.1080/10408444.2019.1579169
EFSA Scientific Committee (2011). Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J 9, 2379. doi:10.2903/j.efsa.2011.2379
Egetenmeyer, N. and Weiss, S. C. (2017). Investigations for the detection of genotoxic substances on TLC plates. J Liq Chromatogr Related Technol 40, 69-74. doi:10.1080/10826076.2017.1284674
European Medicines Agency (1995). ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. CPMP/ICH/381/95, 1-15. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed 06.11.2020)
European Parliament, Committee on the Environment, Public Health and Food Safety (2016). Report on the implementation of the Food Contact Materials Regulation ((EC) No 1935/2004) (2015/2259(INI)). A8-0237/2016, 1-16. https://www.europarl.europa.eu/doceo/document/A-8-2016-0237_EN.pdf
Flückiger-Isler, S. and Kamber, M. (2012). Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat Res Fundam Mol Mech Mutagen 747, 36-45. doi:10.1016/j.mrgentox.2012.03.014
Grob, K., Biedermann, M., Scherbaum, E. et al. (2006). Food contamination with organic materials in perspective: Packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit Rev Food Sci Nutr 46, 529-535. doi:10.1080/10408390500295490
Hamer, B., Bihari, N., Reifferscheid, G. et al. (2000). Evaluation of the SOS/umu-test post-treatment assay for the detection of genotoxic activities of pure compounds and complex environmental mixtures. Mutat Res Genet Toxicol Environ Mutagen 466, 161-171. doi:10.1016/S1383-5718(00)00016-4
ISO – International Organization for Standardization (2000). Determination of the genotoxicity of water and waste water using the umu-test. https://www.iso.org/obp/ui/#iso:std:iso:13829:ed-1:v1:en:sec:A
Jamshidi-Aidji, M. and Morlock, G. E. (2018). Fast equivalency estimation of unknown enzyme inhibitors in situ the effect-directed fingerprint, shown for bacillus lipopeptide extracts. Anal Chem 90, 14260-14268. doi:10.1021/acs.analchem.8b03407
Kirkland, D., Kasper, P., Martus, H.-J. et al. (2016). Updated recommended lists of genotoxic and non-genotoxic chemicals for assessment of the performance of new or improved genotoxicity tests. Mutat Res Genet Toxicol Environ Mutagen 795, 7-30. doi:10.1016/j.mrgentox.2015.10.006
Klingelhöfer, I. and Morlock, G. E. (2014). Sharp-bounded zones link to the effect in planar chromatography-bioassay-mass spectrometry. J Chromatogr A 1360, 288-295. doi:10.1016/j.chroma.2014.07.083
Klingelhöfer, I. and Morlock, G. E. (2015). Bioprofiling of surface/wastewater and bioquantitation of discovered endocrine-active compounds by streamlined direct bioautography. Anal Chem 87, 11098-11104
Klingelhöfer, I., Hockamp, N. and Morlock, G. E. (2020). Non-targeted detection and differentiation of agonists versus antagonists, directly in bioprofiles of everyday products. Anal Chim Acta 125, 288-298. doi:10.1016/j.aca.2020.05.057
Koster, S., Boobis, A. R., Cubberley, R. et al. (2011). Application of the TTC concept to unknown substances found in analysis of foods. Food Chem Toxicol 49, 1643-1660. doi:10.1016/j.fct.2011.03.049
Koster, S., Rennen, M., Leeman, W. et al. (2014). A novel safety assessment strategy for non-intentionally added substances (NIAS) in carton food contact materials. Food Addit Contam 31, 422-443. doi:10.1080/19440049.2013.866718
Koster, S. Bani-Estivals, M., Bonuomo, M. et al. (2015). Guidance on best practices on the risk assessment of non-intentionally added substances (NIAS) in food contact materials and articles. ILSI Europe Report Series, 1-70.
Oda, Y. (2016). Development and progress for three decades in umu test systems. Genes Environ 38, 24. doi:10.1186/s41021-016-0054-8
Rainer, B., Pinter, E., Czerny, T. et al. (2018). Suitability of the Ames test to characterise genotoxicity of food contact material migrates. Food Addit Contam Part A – Chem 35, 2230-2243. doi:10.1080/19440049.2018.1519259
Rainer, B., Mayrhofer, E., Redl, M. et al. (2019). Mutagenicity assessment of food contact material migrates with the Ames MPF assay. Food Addit Contam Part A – Chem 36, 1419-1432. doi:10.1080/19440049.2019.1634841
Reifferscheid, G. and Heil, J. (1996). Validation of the SOS/umu test using test results of 486 chemicals and comparison with the Ames test and carcinogenicity data. Mutat Res Genet Toxicol 369, 129-145. doi:10.1016/S0165-1218(96)90021-X
Schick, D. and Schwack, W. (2017). Planar yeast estrogen screen with resorufin-β-d-galactopyranoside as substrate. J Chromatogr A 1497, 155-163. doi:10.1016/j.chroma.2017.03.047
Schilter, B., Burnett, K., Eskes, C. et al. (2019). Value and limitation of in vitro bioassays to support the application of the threshold of toxicological concern to prioritise unidentified chemicals in food contact materials. Food Addit Contam 36, 1903-1936. doi:10.1080/19440049.2019.1664772
Shakibai, D., Riegraf, C., Moscovici, L. et al. (2019). Coupling high-performance thin-layer chromatography with bacterial genotoxicity bioreporters. Environ Sci Technol 53, 6410-6419. doi:10.1021/acs.est.9b00921
Stütz, L., Leitner, P., Schulz, W. et al. (2019). Identification of genotoxic transformation products by effect-directed analysis with high-performance thin-layer chromatography and non-target screening. JPC-J Planar Chromatogr 32, 173-182. doi:10.1556/1006.2019.32.3.1
Veyrand, J., Marin-Kuan, M. and Bezencon, C. et al. (2017). Integrating bioassays and analytical chemistry as an improved approach to support safety assessment of food contact materials. Food Addit Contam 34, 1807-1816. doi:10.1080/19440049.2017.1358466
Wöhrmann, U. (2019). Entwicklung einer HPTLC-basierten Nachweismethode gentoxischer Substanzen durch Salmonella typhimurium. Master thesis, Justus Liebig University Giessen.
Yüce, I., Mayr, M. and Morlock, G. E. (2019). Quantitative inkjet application on self-printed, binder-free HPTLC layers for submicromole-scaled analytical 1H NMR spectroscopy. Anal Chim Acta 1087, 131-139. doi:10.1016/j.aca.2019.08.013