TBBPA targets converging key events of human oligodendrocyte development resulting in two novel AOPs
Main Article Content
Abstract
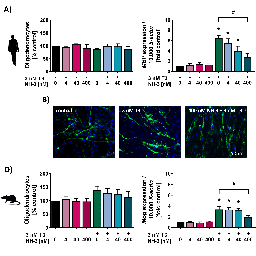
Myelinating oligodendrocytes (OLs) establish saltatory nerve conduction during white matter development. Thus, interference with oligodendrogenesis leads to an adverse outcome on brain performance in the child due to aberrant myelination. An intertwined network of hormonal, transcriptional and biosynthetic processes regulates OL development, thereby simultaneously creating various routes of interference for environmental toxicants. The flame retardant tetrabromobisphenol A (TBBPA) is debated as an endocrine disruptor, especially of the thyroid hormone (TH) system. We identified how TBBPA interferes with the establishment of a population of maturing OLs by two independent modes-of-action (MoA), dependent and independent of TH signaling. Combining the previously published oligodendrocyte maturation assay (NPC6) with large-scale transcriptomics, we describe TBBPA as a TH disruptor, impairing human OL maturation in vitro by dysregulation of oligodendrogenesis-associated genes (i.e., MBP, KLF9 and EGR1). Furthermore, TBBPA disrupts a gene expression network regulating cholesterol homeostasis, reducing OL numbers independently of TH signaling. These two MoA converge in a novel putative adverse outcome pathway (AOP) network on the key event (KE) hypomyelination. Comparative analyses of human and rat neural progenitor cells (NPCs) revealed that human oligodendrogenesis is more sensitive to endocrine disruption by TBBPA. Therefore, ethical, cost-efficient and species-overarching in vitro assays are needed for developmental neurotoxicity hazard assessment. By incorporation of large-scale transcriptomic analyses, we brought the NPC6 assay to a higher readiness level for future applications in a regulatory context. The combination of phenotypic and transcriptomic analyses helps to study MoA to eventually build AOPs for a better understanding of neurodevelopmental toxicity.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Abdallah, M. A. and Harrad, S. (2011). Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environ Int 37, 443-448. doi:10.1016/j.envint.2010.11.008
Annunziata, P., Federico, A., D’Amore, I. et al. (1983). Impairment of human brain development: Glycoconjugate and lipid changes in congenital athyroidism. Early Hum Dev 8, 269-278. doi:10.1016/0378-3782(83)90009-9
Aung, H. H., Altman, R., Nyunt, T. et al. (2016). Lipotoxic brain microvascular injury is mediated by activating transcription factor 3-dependent inflammatory and oxidative stress pathways. J Lipid Res 57, 955-968. doi:10.1194/jlr.M061853
Baas, D., Bourbeau, D., Sarlieve, L. L. et al. (1997). Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia 19, 324-332. doi:10.1002/(sici)1098-1136(199704)19:4<324::aid-glia5>3.0.co;2-x
Back, S. A., Luo, N. L., Borenstein, N. S. et al. (2001). Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21, 1302-1312.
Bal-Price, A., Crofton, K. M., Leist, M. et al. (2015). International stakeholder network (ISTNET): Creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol 89, 269-287. doi:10.1007/s00204-015-1464-2
Bal-Price, A., Hogberg, H. T., Crofton, K. M. et al. (2018). Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX 35, 306-352. doi:10.14573/altex.1712081
Barateiro, A., Brites, D. and Fernandes, A. (2016). Oligodendrocyte development and myelination in neurodevelopment: Molecular mechanisms in health and disease. Curr Pharm Des 22, 656-679. doi:10.2174/1381612822666151204000636
Barenys, M., Gassmann, K., Baksmeier, C. et al. (2017). Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch Toxicol 91, 827-837. doi:10.1007/s00204-016-1709-8
Bauer, A. J. and Wassner, A. J. (2019). Thyroid hormone therapy in congenital hypothyroidism and pediatric hypothyroidism. Endocrine 66, 51-62. doi:10.1007/s12020-019-02024-6
Baumann, J., Barenys, M., Gassmann, K. et al. (2014). Comparative human and rat “neurosphere assay” for developmental neurotoxicity testing. Curr Protoc Toxicol 59, 12.21.11-24. doi:10.1002/0471140856.tx1221s59
Baumann, J., Gassmann, K., Masjosthusmann, S. et al. (2016). Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch Toxicol 90, 1415-1427. doi:10.1007/s00204-015-1568-8
Bell, S. M., Phillips, J., Sedykh, A. et al. (2017). An integrated chemical environment to support 21st-century toxicology. Environ Health Perspect 125, 054501. doi:10.1289/EHP1759
Berghoff, S. A., Gerndt, N., Winchenbach, J. et al. (2017). Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat Commun 8, 14241. doi:10.1038/ncomms14241
Bezine, M., Debbabi, M., Nury, T. et al. (2017). Evidence of K+ homeostasis disruption in cellular dysfunction triggered by 7-ketocholesterol, 24S-hydroxycholesterol, and tetracosanoic acid (C24:0) in 158N murine oligodendrocytes. Chem Phys Lipids 207, 135-150. doi:10.1016/j.chemphyslip.2017.03.006
Billon, N., Jolicoeur, C., Tokumoto, Y. et al. (2002). Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRalpha1). EMBO J 21, 6452-6460. doi:10.1093/emboj/cdf662
Blasi, F., Ciarrocchi, A., Luddi, A. et al. (2002). Stage-specific gene expression in early differentiating oligodendrocytes. Glia 39, 114-123. doi:10.1002/glia.10092
Bolstad, B. M., Irizarry, R. A., Astrand, M. et al. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185-193. doi:10.1093/bioinformatics/19.2.185
Braun, J. M., Buckley, J. P., Cecil, K. M. et al. (2020). Adolescent follow-up in the health outcomes and measures of the environment (home) study: Cohort profile. BMJ Open 10, e034838. doi:10.1136/bmjopen-2019-034838
Breier, J. M., Gassmann, K., Kayser, R. et al. (2010). Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: State of the science. Neurotoxicol Teratol 32, 4-15. doi:10.1016/j.ntt.2009.06.005
Caporali, P., Bruno, F., Palladino, G. et al. (2016). Developmental delay in motor skill acquisition in Niemann-Pick C1 mice reveals abnormal cerebellar morphogenesis. Acta Neuropathol Commun 4, 94. doi:10.1186/s40478-016-0370-z
Cariou, R., Antignac, J. P., Zalko, D. et al. (2008). Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 73, 1036-1041. doi:10.1016/j.chemosphere.2008.07.084
Chen, J., Tanguay, R. L., Xiao, Y. et al. (2016). TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ Pollut 216, 53-63. doi:10.1016/j.envpol.2016.05.059
Cheng, X., Zheng, Y., Bu, P. et al. (2018). Apolipoprotein E as a novel therapeutic neuroprotection target after traumatic spinal cord injury. Exp Neurol 299, 97-108. doi:10.1016/j.expneurol.2017.10.014
Cho, J. H., Lee, S., Jeon, H. et al. (2020). Tetrabromobisphenol A-induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction. Neurotox Res 38, 74-85. doi:10.1007/s12640-020-00179-z
Cope, R. B., Kacew, S. and Dourson, M. (2015). A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague-Dawley rats. Toxicology 329, 49-59. doi:10.1016/j.tox.2014.12.013
Coperchini, F., Awwad, O., Rotondi, M. et al. (2017). Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest 40, 105-121. doi:10.1007/s40618-016-0572-z
Coughtrie, M. W., Burchell, B., Leakey, J. E. et al. (1988). The inadequacy of perinatal glucuronidation: Immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol 34, 729-735.
Cui, X., Chopp, M., Zhang, Z. et al. (2017). ABCA1/ApoE/HDL pathway mediates GW3965-induced neurorestoration after stroke. Stroke 48, 459-467. doi:10.1161/STROKEAHA.116.015592
Dach, K., Bendt, F., Huebenthal, U. et al. (2017). BDE-99 impairs differentiation of human and mouse NPCs into the oligodendroglial lineage by species-specific modes of action. Sci Rep 7, 44861. doi:10.1038/srep44861
de Escobar, G. M., Obregon, M. J. and del Rey, F. E. (2004). Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18, 225-248. doi:10.1016/j.beem.2004.03.012
Denver, R. J. and Williamson, K. E. (2009). Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology 150, 3935-3943. doi:10.1210/en.2009-0050
Dohi, E., Choi, E. Y., Rose, I. V. L. et al. (2017). Behavioral changes in mice lacking interleukin-33. eNeuro 4, e0147. doi:10.1523/ENEURO.0147-17.2017
Duclot, F. and Kabbaj, M. (2017). The role of early growth response 1 (EGR1) in brain plasticity and neuropsychiatric disorders. Front Behav Neurosci 11, 35. doi:10.3389/fnbeh.2017.00035
Dugas, J. C., Ibrahim, A. and Barres, B. A. (2012). The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci 50, 45-57. doi:10.1016/j.mcn.2012.03.007
Emery, B. (2010). Regulation of oligodendrocyte differentiation and myelination. Science 330, 779-782. doi:10.1126/science.1190927
Farsetti, A., Desvergne, B., Hallenbeck, P. et al. (1992). Characterization of myelin basic protein thyroid hormone response element and its function in the context of native and heterologous promoter. J Biol Chem 267, 15784-15788.
Gan, H. T., Tham, M., Hariharan, S. et al. (2011). Identification of ApoE as an autocrine/paracrine factor that stimulates neural stem cell survival via MAPK/ERK signaling pathway. J Neurochem 117, 565-578. doi:10.1111/j.1471-4159.2011.07227.x
Ghorbel, M. T., Seugnet, I., Hadj-Sahraoui, N. et al. (1999). Thyroid hormone effects on Krox-24 transcription in the post-natal mouse brain are developmentally regulated but are not correlated with mitosis. Oncogene 18, 917-924. doi:10.1038/sj.onc.1202378
Gika, A. D., Siddiqui, A., Hulse, A. J. et al. (2010). White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med Child Neurol 52, 475-482. doi:10.1111/j.1469-8749.2009.03471.x
Groeneweg, S., Peeters, R. P., Moran, C. et al. (2019). Effectiveness and safety of the tri-iodothyronine analogue triac in children and adults with MCT8 deficiency: An international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7, 695-706. doi:10.1016/S2213-8587(19)30155-X
Gruters, A. and Krude, H. (2011). Detection and treatment of congenital hypothyroidism. Nat Rev Endocrinol 8, 104-113. doi:10.1038/nrendo.2011.160
Gupta, R. K., Bhatia, V., Poptani, H. et al. (1995). Brain metabolite changes on in vivo proton magnetic resonance spectroscopy in children with congenital hypothyroidism. J Pediatr 126, 389-392. doi:10.1016/s0022-3476(95)70454-x
Haddow, J. E., Palomaki, G. E., Allan, W. C. et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341, 549-555. doi:10.1056/NEJM199908193410801
Hamers, T., Kamstra, J. H., Sonneveld, E. et al. (2006). In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92, 157-173. doi:10.1093/toxsci/kfj187
Haq, E., Giri, S., Singh, I. et al. (2003). Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem 86, 1428-1440. doi:10.1046/j.1471-4159.2003.01941.x
Ibarrola, N. and Rodriguez-Pena, A. (1997). Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res 752, 285-293. doi:10.1016/s0006-8993(96)01480-1
Kawamura, A., Katayama, Y., Nishiyama, M. et al. (2020). Oligodendrocyte dysfunction due to Chd8 mutation gives rise to behavioral deficits in mice. Hum Mol Genet 29, 1274-1291. doi:10.1093/hmg/ddaa036
Kim, B., Colon, E., Chawla, S. et al. (2015). Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ Health 14, 64. doi:10.1186/s12940-015-0051-6
Kim, U. J. and Oh, J. E. (2014). Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ Pollut 184, 193-200. doi:10.1016/j.envpol.2013.08.034
Kitamura, S., Jinno, N., Ohta, S. et al. (2002). Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun 293, 554-559. doi:10.1016/S0006-291X(02)00262-0
Kitamura, S., Kato, T., Iida, M. et al. (2005). Anti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: Affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci 76, 1589-1601. doi:10.1016/j.lfs.2004.08.030
La Piana, R., Vanasse, M., Brais, B. et al. (2015). Myelination delay and Allan-Herndon-Dudley syndrome caused by a novel mutation in the SLC16A2 gene. J Child Neurol 30, 1371-1374. doi:10.1177/0883073814555189
Lee, J. Y. and Petratos, S. (2016). Thyroid hormone signaling in oligodendrocytes: From extracellular transport to intracellular signal. Mol Neurobiol 53, 6568-6583. doi:10.1007/s12035-016-0013-1
Lenters, V., Iszatt, N., Forns, J. et al. (2019). Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ Int 125, 33-42. doi:10.1016/j.envint.2019.01.020
Leonetti, C., Butt, C. M., Hoffman, K. et al. (2016). Brominated flame retardants in placental tissues: Associations with infant sex and thyroid hormone endpoints. Environ Health 15, 113. doi:10.1186/s12940-016-0199-8
Liang, S., Liang, S., Zhou, H. et al. (2019). Typical halogenated flame retardants affect human neural stem cell gene expression during proliferation and differentiation via glycogen synthase kinase 3 beta and T3 signaling. Ecotoxicol Environ Saf 183, 109498. doi:10.1016/j.ecoenv.2019.109498
Lilienthal, H., Verwer, C. M., van der Ven, L. T. et al. (2008). Exposure to tetrabromobisphenol a (TBBPA) in Wistar rats: Neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology 246, 45-54. doi:10.1016/j.tox.2008.01.007
Lin, C. Y., Wen, L. L., Lin, L. Y. et al. (2013). The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J Hazard Mater 244-245, 637-644. doi:10.1016/j.jhazmat.2012.10.049
Malliari, E. and Kalantzi, O. I. (2017). Children’s exposure to brominated flame retardants in indoor environments – A review. Environ Int 108, 146-169. doi:10.1016/j.envint.2017.08.011
Masjosthusmann, S., Becker, D., Petzuch, B. et al. (2018). A transcriptome comparison of time-matched developing human, mouse and rat neural progenitor cells reveals human uniqueness. Toxicol Appl Pharmacol 354, 40-55. doi:10.1016/j.taap.2018.05.009
Masjosthusmann, S., Siebert, C., Hubenthal, U. et al. (2019). Arsenite interrupts neurodevelopmental processes of human and rat neural progenitor cells: The role of reactive oxygen species and species-specific antioxidative defense. Chemosphere 235, 447-456. doi:10.1016/j.chemosphere.2019.06.123
Mathews, E. S., Mawdsley, D. J., Walker, M. et al. (2014). Mutation of 3-hydroxy-3-methylglutaryl CoA synthase I reveals requirements for isoprenoid and cholesterol synthesis in oligodendrocyte migration arrest, axon wrapping, and myelin gene expression. J Neurosci 34, 3402-3412. doi:10.1523/JNEUROSCI.4587-13.2014
Mathews, E. S. and Appel, B. (2016). Cholesterol biosynthesis supports myelin gene expression and axon ensheathment through modulation of P13K/Akt/mTOR signaling. J Neurosci 36, 7628-7639. doi:10.1523/JNEUROSCI.0726-16.2016
Matsukami, H., Tue, N. M., Suzuki, G. et al. (2015). Flame retardant emission from e-waste recycling operation in northern vietnam: Environmental occurrence of emerging organophosphorus esters used as alternatives for PBDEs. Sci Total Environ 514, 492-499. doi:10.1016/j.scitotenv.2015.02.008
Mewar, R. and McMorris, F. A. (1997). Expression of insulin-like growth factor-binding protein messenger RNAs in developing rat oligodendrocytes and astrocytes. J Neurosci Res 50, 721-728. doi:10.1002/(SICI)1097-4547(19971201)50:5<721::AID-JNR9>3.0.CO;2-J
Moors, M., Rockel, T. D., Abel, J. et al. (2009). Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117, 1131-1138. doi:10.1289/ehp.0800207
Mughal, B. B., Fini, J. B. and Demeneix, B. A. (2018). Thyroid-disrupting chemicals and brain development: An update. Endocr Connect 7, R160-R186. doi:10.1530/EC-18-0029
Murray, K. and Dubois-Dalcq, M. (1997). Emergence of oligodendrocytes from human neural spheres. J Neurosci Res 50, 146-156. doi:10.1002/(SICI)1097-4547(19971015)50:2<146::AID-JNR4>3.0.CO;2-F
Nakajima, A., Saigusa, D., Tetsu, N. et al. (2009). Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol Lett 189, 78-83. doi:10.1016/j.toxlet.2009.05.003
Nelissen, K., Mulder, M., Smets, I. et al. (2012). Liver X receptors regulate cholesterol homeostasis in oligodendrocytes. J Neurosci Res 90, 60-71. doi:10.1002/jnr.22743
Nguyen, N. H., Apriletti, J. W., Cunha Lima, S. T. et al. (2002). Rational design and synthesis of a novel thyroid hormone antagonist that blocks coactivator recruitment. J Med Chem 45, 3310-3320. doi:10.1021/jm0201013
Ni, H. G. and Zeng, H. (2013). HBCD and TBBPA in particulate phase of indoor air in Shenzhen, China. Sci Total Environ 458-460, 15-19. doi:10.1016/j.scitotenv.2013.04.003
Nimtz, L., Klose, J., Masjosthusmann, S. et al. (2019). The neurosphere assay as an in vitro method for developmental neurotoxicity (DNT) evaluation. In M. Aschner and L. Costa (eds.), Cell Culture Techniques (141-168). Neuromethods. Vol. 145. New York, NY, USA: Humana. doi:10.1007/978-1-4939-9228-7_8
Norton, W. T. and Poduslo, S. E. (1973). Myelination in rat brain: Changes in myelin composition during brain maturation. J Neurochem 21, 759-773. doi:10.1111/j.1471-4159.1973.tb07520.x
Noyes, P. D., Haggard, D. E., Gonnerman, G. D. et al. (2015). Advanced morphological – Behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci 145, 177-195. doi:10.1093/toxsci/kfv044
Noyes, P. D., Friedman, K. P., Browne, P. et al. (2019). Evaluating chemicals for thyroid disruption: Opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect 127, 95001. doi:10.1289/EHP5297
Parsons, A., Lange, A., Hutchinson, T. H. et al. (2019). Molecular mechanisms and tissue targets of brominated flame retardants, BDE-47 and TBBPA, in embryo-larval life stages of zebrafish (Danio rerio). Aquat Toxicol 209, 99-112. doi:10.1016/j.aquatox.2019.01.022
Patel, J., Landers, K., Li, H. et al. (2011). Thyroid hormones and fetal neurological development. J Endocrinol 209, 1-8. doi:10.1530/JOE-10-0444
Pfrieger, F. W. and Ungerer, N. (2011). Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res 50, 357-371. doi:10.1016/j.plipres.2011.06.002
Przybyla, J., Geldhof, G. J., Smit, E. et al. (2018). A cross sectional study of urinary phthalates, phenols and perchlorate on thyroid hormones in us adults using structural equation models (NHANES 2007-2008). Environ Res 163, 26-35. doi:10.1016/j.envres.2018.01.039
Rock, K. D., Gillera, S. E. A., Devarasetty, P. et al. (2019). Sex-specific behavioral effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats. Neurotoxicology 75, 136-147. doi:10.1016/j.neuro.2019.09.003
Rovet, J. and Daneman, D. (2003). Congenital hypothyroidism: A review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs 5, 141-149. doi:10.2165/00128072-200305030-00001
Saegusa, Y., Fujimoto, H., Woo, G. H. et al. (2009). Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol 28, 456-467. doi:10.1016/j.reprotox.2009.06.011
Safina, D., Schlitt, F., Romeo, R. et al. (2016). Low-density lipoprotein receptor-related protein 1 is a novel modulator of radial glia stem cell proliferation, survival, and differentiation. Glia 64, 1363-1380. doi:10.1002/glia.23009
Saher, G., Rudolphi, F., Corthals, K. et al. (2012). Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat Med 18, 1130-1135. doi:10.1038/nm.2833
Saher, G. and Stumpf, S. K. (2015). Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851, 1083-1094. doi:10.1016/j.bbalip.2015.02.010
Sarret, C., Oliver Petit, I. and Tonduti, D. (1993). Allan-Herndon-Dudley syndrome. In M. P. Adam, H. H. Ardinger, R. A. Pagon et al. (eds.), GeneReviews®. Seattle, WA, USA: University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/pubmed/20301789
Schauer, U. M., Volkel, W. and Dekant, W. (2006). Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol Sci 91, 49-58. doi:10.1093/toxsci/kfj132
Schmuck, M. R., Temme, T., Dach, K. et al. (2017). Omnisphero: A high-content image analysis (HCA) approach for phenotypic developmental neurotoxicity (DNT) screenings of organoid neurosphere cultures in vitro. Arch Toxicol 91, 2017-2028. doi:10.1007/s00204-016-1852-2
Schoonover, C. M., Seibel, M. M., Jolson, D. M. et al. (2004). Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology 145, 5013-5020. doi:10.1210/en.2004-0065
Scobie, K. N., Hall, B. J., Wilke, S. A. et al. (2009). Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci 29, 9875-9887. doi:10.1523/JNEUROSCI.2260-09.2009
Shi, Z., Zhang, L., Zhao, Y. et al. (2017). A national survey of tetrabromobisphenol-A, hexabromocyclododecane and decabrominated diphenyl ether in human milk from China: Occurrence and exposure assessment. Sci Total Environ 599-600, 237-245. doi:10.1016/j.scitotenv.2017.04.237
Singh, L., Pressly, B., Mengeling, B. J. et al. (2016). Chasing the elusive benzofuran impurity of the THR antagonist NH-3: Synthesis, isotope labeling, and biological activity. J Org Chem 81, 1870-1876. doi:10.1021/acs.joc.5b02665
Sjodin, A., Patterson, D. G., Jr. and Bergman, A. (2003). A review on human exposure to brominated flame retardants – Particularly polybrominated diphenyl ethers. Environ Int 29, 829-839. doi:10.1016/S0160-4120(03)00108-9
Sock, E., Leger, H., Kuhlbrodt, K. et al. (1997). Expression of Krox proteins during differentiation of the O-2A progenitor cell line CG-4. J Neurochem 68, 1911-1919. doi:10.1046/j.1471-4159.1997.68051911.x
Stoll, G., Meuller, H. W., Trapp, B. D. et al. (1989). Oligodendrocytes but not astrocytes express apolipoprotein E after injury of rat optic nerve. Glia 2, 170-176. doi:10.1002/glia.440020306
Sung, H. Y., Chen, W. Y., Huang, H. T. et al. (2019). Down-regulation of interleukin-33 expression in oligodendrocyte precursor cells impairs oligodendrocyte lineage progression. J Neurochem 150, 691-708. doi:10.1111/jnc.14788
Takigami, H., Suzuki, G., Hirai, Y. et al. (2009). Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere 76, 270-277. doi:10.1016/j.chemosphere.2009.03.006
Takikita, S., Fukuda, T., Mohri, I. et al. (2004). Perturbed myelination process of premyelinating oligodendrocyte in Niemann-Pick type C mouse. J Neuropathol Exp Neurol 63, 660-673. doi:10.1093/jnen/63.6.660
Topilko, P., Levi, G., Merlo, G. et al. (1997). Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res 50, 702-712. doi:10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L
Valenza, M., Marullo, M., Di Paolo, E. et al. (2015). Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington’s disease. Cell Death Differ 22, 690-702. doi:10.1038/cdd.2014.162
Van der Ven, L. T., Van de Kuil, T., Verhoef, A. et al. (2008). Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology 245, 76-89. doi:10.1016/j.tox.2007.12.009
van Tilborg, E., de Theije, C. G. M., van Hal, M. et al. (2018). Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 66, 221-238. doi:10.1002/glia.23256
Veereman-Wauters, G. (1996). Neonatal gut development and postnatal adaptation. Eur J Pediatr 155, 627-632. doi:10.1007/BF01957141
Wages, P. A., Joshi, P., Tallman, K. A. et al. (2020). Screening toxcast for chemicals that affect cholesterol biosynthesis: Studies in cell culture and human induced pluripotent stem cell-derived neuroprogenitors. Environ Health Perspect 128, 17014. doi:10.1289/EHP5053
Walter, K. M., Dach, K., Hayakawa, K. et al. (2019). Ontogenetic expression of thyroid hormone signaling genes: An in vitro and in vivo species comparison. PLoS One 14, e0221230. doi:10.1371/journal.pone.0221230
Wang, X., Li, R., Zacharek, A. et al. (2018). Administration of downstream ApoE attenuates the adverse effect of brain ABCA1 deficiency on stroke. Int J Mol Sci 19, doi:10.3390/ijms19113368
Wang, Y., Li, Y., Qin, Z. et al. (2017). Re-evaluation of thyroid hormone signaling antagonism of tetrabromobisphenol A for validating the T3-induced Xenopus metamorphosis assay. J Environ Sci (China) 52, 325-332. doi:10.1016/j.jes.2016.09.021
Wei, W., Wang, Y., Dong, J. et al. (2015). Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ Toxicol 30, 1264-1274. doi:10.1002/tox.21997
Wetmore, B. A., Wambaugh, J. F., Ferguson, S. S. et al. (2012). Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 125, 157-174. doi:10.1093/toxsci/kfr254
Xiao, C., Grandjean, P., Valvi, D. et al. (2020). Associations of exposure to perfluoroalkyl substances with thyroid hormone concentrations and birth size. J Clin Endocrinol Metab 105, doi:10.1210/clinem/dgz147
Yang, S., Wang, S., Liu, H. et al. (2012). Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ Sci Pollut Res Int 19, 4090-4096. doi:10.1007/s11356-012-1023-9
Yasuda, K., Maki, T., Kinoshita, H. et al. (2020). Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res 46, 101866. doi:10.1016/j.scr.2020.101866
Yeung, M. S., Zdunek, S., Bergmann, O. et al. (2014). Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766-774. doi:10.1016/j.cell.2014.10.011
Zhang, Y. F., Xu, W., Lou, Q. Q. et al. (2014). Tetrabromobisphenol A disrupts vertebrate development via thyroid hormone signaling pathway in a developmental stage-dependent manner. Environ Sci Technol 48, 8227-8234. doi:10.1021/es502366g
Zhou, Y., Bazick, H., Miles, J. R. et al. (2019). A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice. Exp Neurol 321, 113031. doi:10.1016/j.expneurol.2019.113031
Zhu, B., Zhao, G., Yang, L. et al. (2018). Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere 197, 353-361. doi:10.1016/j.chemosphere.2018.01.080
Zoeller, R. T. (2007). Environmental chemicals impacting the thyroid: Targets and consequences. Thyroid 17, 811-817. doi:10.1089/thy.2007.0107