Academic application of Good Cell Culture Practice for induced pluripotent stem cells
Main Article Content
Abstract
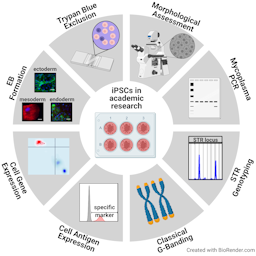
Human induced pluripotent stem cells (hiPSC) are a promising tool for replacing animal-based experiments. To warrant data reproducibility, quality-controlled research material is recommended. While the need for global harmonization of quality standards for stem cell banking centers, commercial providers, pre-clinical and clinical use of cells is well documented, there are no recommendations available for quality control of hiPSC in an academic research environment to date. To fill this gap, we here give an example of a quality-controlled, two-tiered banking process producing a fully characterized master cell bank (MCB) and partially characterized respective working cell banks (WCB). Characterization includes the study of morphology, mycoplasma contamination, cell line identity, karyotype stability, cell antigen expression and viability, gene expression, pluripotency, and post-thaw recovery. Costs of these procedures are calculated. We present the results of the proposed testing panel of two hiPSC MCBs and show that both fulfil the defined specifications regarding the above-mentioned characterization assays during and upon banking. In conclusion, we propose a panel of eight assays, which are practical and useful for an academic research laboratory working with hiPSCs. Meeting these proposed specifications ensures the quality of pluripotent stem cells throughout diverse experiments at moderate costs.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Abbot, S., Agbanyo, F., Ahlfors, J. E. et al. (2018). Report of the international conference on manufacturing and testing of pluripotent stem cells. In Biologicals (67–83). Academic Press. doi:10.1016/j.biologicals.2018.08.004
Adler, S., Allsopp, T., Bremer, S. et al. (2007). hESC Technology for Toxicology and Drug Development: Summary of Current Status and Recommendations for Best Practice and Standardization The Report and Recommendations of and ECVAM Workshop
Almeida, J. L., Cole, K. D. and Plant, A. L. (2016). Standards for Cell Line Authentication and Beyond. PLoS Biol 14. doi:10.1371/journal.pbio.1002476
Amps, K., Andrews, P. W., Anyfantis, G. et al. (2011). Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol 29, 1132–1144. doi:10.1038/nbt.2051
Andrews, P. W., Baker, D., Benvinisty, N. et al. (2015). Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen Med 10, 1–44. doi:10.2217/rme.14.93
Archibald, P. R. T., Chandra, A., Thomas, D. et al. (2016). Comparability of automated human induced pluripotent stem cell culture: a pilot study. Bioprocess Biosyst Eng 39, 1847–1858. doi:10.1007/s00449-016-1659-9
Armstrong, S. E., Mariano, J. A. and Lundin, D. J. (2010). The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals 38, 211–213. doi:10.1016/j.biologicals.2010.03.002
Assou, S., Bouckenheimer, J. and De Vos, J. (2018). Concise Review: Assessing the Genome Integrity of Human Induced Pluripotent Stem Cells: What Quality Control Metrics? Stem Cells 36, 814–821. doi:10.1002/stem.2797
Baghbaderani, B. A., Tian, X., Neo, B. H. et al. (2015). CGMP-manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Reports 5, 647–659. doi:10.1016/j.stemcr.2015.08.015
Bai, Q., Desprat, R., Klein, B. et al. (2013). Embryonic Stem Cells or Induced Pluripotent Stem Cells? A DNA Integrity Perspective. Curr Gene Ther 13, 93–98. doi:10.2174/1566523211313020003
Bairoch, A. (2018). The cellosaurus, a cell-line knowledge resource. J Biomol Tech 29, 25–38. doi:10.7171/jbt.18-2902-002
Baker, D. E. C., Harrison, N. J., Maltby, E. et al. (2007). Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol 25, 207–215. doi:10.1038/nbt1285
Baker, M. (2016). How quality control could save your science. Nature 529, 456–458. doi:10.1038/529456a
Baker, M. and Penny, D. (2016). Is there a reproducibility crisis? Nature 533, 452–454. doi:10.1038/533452A
Bartalucci, N., Romagnoli, S., Contini, E. et al. (2019). Long Reads, Short Time: Feasibility of Prenatal Sample Karyotyping by Nanopore Genome Sequencing. Clin Chem 65, 1605–1608. doi:10.1373/clinchem.2019.310805
Bickmore, W. A. (2001). Karyotype Analysis and Chromosome Banding. In Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1038/npg.els.0001160
Bock, C., Kiskinis, E., Verstappen, G. et al. (2011). Reference maps of human es and ips cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452. doi:10.1016/j.cell.2010.12.032
Borowiak, M., Maehr, R., Chen, S. et al. (2009). Small Molecules Efficiently Direct Endodermal Differentiation of Mouse and Human Embryonic Stem Cells. Cell Stem Cell 4, 348–358. doi:10.1016/j.stem.2009.01.014
Bruchmüller, I., Pirkl, E., Herrmann, R. et al. (2006). Introduction of a validation concept for a PCR-based Mycoplasma detection assay. Cytotherapy 8, 62–69. doi:10.1080/14653240500518413
Buehring, G. C., Eby, E. A. and Eby, M. J. (2004). Cell line cross-contamination: How aware are mammalian cell culturists of the problem and how to monitor it? Vitr Cell Dev Biol - Anim 40, 211–215. doi:10.1290/1543-706x(2004)40<211:clchaa>2.0.co;2
Burridge, P. W., Keller, G., Gold, J. D. et al. (2012). Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28. doi:10.1016/j.stem.2011.12.013
Buta, C., David, R., Dressel, R. et al. (2013). Reconsidering pluripotency tests: Do we still need teratoma assays? Stem Cell Res 11, 552–562. doi:10.1016/j.scr.2013.03.001
Chambers, S. M., Fasano, C. A., Papapetrou, E. P. et al. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27, 275–280. doi:10.1038/nbt.1529
Chi, K. R. (2013). Out, Damned Mycoplasma! Pointers for keeping your cell culture free of mycoplasma contamination. Sci. Available at: https://www.the-scientist.com/?articles.view/articleNo/38381/[Accessed January 13, 2021].
Christensen, K., Roudnicky, F., Patsch, C. et al. (2018). Requirements for using IPSC-based cell models for assay development in drug discovery. In Advances in Biochemical Engineering/Biotechnology (207–220). Springer Science and Business Media Deutschland GmbH. doi:10.1007/10_2017_23
Coecke, S., Balls, M., Bowe, G. et al. (2005). Guidance on good cell culture practice: A Report of the Second ECVAM Task Force on good cell culture practice. ATLA Altern to Lab Anim 33, 261–287. doi:10.1177/026119290503300313
Conrad, D. F., Pinto, D., Redon, R. et al. (2010). Origins and functional impact of copy number variation in the human genome. Nature 464, 704–712. doi:10.1038/nature08516
Crook, J. M., Tomaskovic-Crook, E. and Ludwig, T. E. (2017). Cryobanking Pluripotent Stem Cells. Methods Mol Biol 1590, 151–164. doi:10.1007/978-1-4939-6921-0_11
D’Antonio, M., Woodruff, G., Nathanson, J. L. et al. (2017). High-Throughput and Cost-Effective Characterization of Induced Pluripotent Stem Cells. Stem Cell Reports 8, 1101–1111. doi:10.1016/j.stemcr.2017.03.011
Dekant, W. (2016). Toxicology and the reproducibility crisis: Scientific publishing, hazard assessment and risk characterization. Toxicol Lett 263, 76–77. doi:10.1016/j.toxlet.2016.09.001
Dirks, W. G., MacLeod, R. A. F., Nakamura, Y. et al. (2010). Cell line cross-contamination initiative: An interactive reference database of STR profiles covering common cancer cell lines. Int J Cancer 126, 303–304. doi:10.1002/ijc.24999
Drexler, H. G. and Uphoff, C. C. (2002). Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. In Cytotechnology (75–90). Cytotechnology. doi:10.1023/A:1022913015916
Freedman, L. P., Gibson, M. C., Ethier, S. P. et al. (2015). Reproducibility: Changing the policies and culture of cell line authentication. Nat Methods 12, 493–497. doi:10.1038/nmeth.3403
Fritsche, E., Haarmann-Stemmann, T., Kapr, J. et al. (2020). Stem Cells for Next Level Toxicity Testing in the 21st Century. Small. doi:10.1002/smll.202006252
Gertow, K., Przyborski, S., Loring, J. F. et al. (2007). Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol Chapter 1. doi:10.1002/9780470151808.sc01b04s3
Gropp, M., Shilo, V., Vainer, G. et al. (2012). Standardization of the Teratoma Assay for Analysis of Pluripotency of Human ES Cells and Biosafety of Their Differentiated Progeny. PLoS One 7. doi:10.1371/journal.pone.0045532
Hay, R. J., Macy, M. L. and Chen, T. R. (1989). Mycoplasma infection of cultured cells. Nature 339, 487–488. doi:10.1038/339487a0
Hook, E. B. (1977). Exclusion of chromosomal mosaicism: tables of 90%, 95%, and 99% confidence limits and comments on use. Am J Hum Genet 29, 94–97.
Howe, B., Umrigar, A. and Tsien, F. (2014). Chromosome Preparation From Cultured Cells. J Vis Exp, 50203. doi:10.3791/50203
Hughes, P., Marshall, D., Reid, Y. et al. (2007). The costs of using unauthenticated, over-passaged cell lines: How much more data do we need? Biotechniques 43, 575–586. doi:10.2144/000112598
ISCBI (2009). Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev Reports 5, 301–314. doi:10.1007/s12015-009-9085-x
ISCI (2018). Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nat Commun 9, 1925. doi:10.1038/s41467-018-04011-3
ISCN (2016). An International System for Human Cytogenomic Nomenclature. J. McGowan-Jordan, A. Simons and M. Schmid (eds.),. S. Karger AG. doi:10.1159/isbn.978-3-318-06861-0
Kattman, S. J., Witty, A. D., Gagliardi, M. et al. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240. doi:10.1016/j.stem.2010.12.008
Kilpinen, H., Goncalves, A., Leha, A. et al. (2017). Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 546, 370–375. doi:10.1038/nature22403
Kim, J. H., Alderton, A., Crook, J. M. et al. (2019). A Report from a Workshop of the International Stem Cell Banking Initiative, Held in Collaboration of Global Alliance for iPSC Therapies and the Harvard Stem Cell Institute, Boston, 2017. In Stem Cells (1130–1135). Wiley-Blackwell. doi:10.1002/stem.3003
Kim, J. H., Kurtz, A., Yuan, B. Z. et al. (2017). Report of the International Stem Cell Banking Initiative Workshop Activity: Current Hurdles and Progress in Seed-Stock Banking of Human Pluripotent Stem Cells. In Stem Cells Translational Medicine (1956–1962). John Wiley and Sons Ltd. doi:10.1002/sctm.17-0144
Kleensang, A., Vantangoli, M. M., Odwin-Dacosta, S. et al. (2016). Genetic variability in a frozen batch of MCF-7 cells invisible in routine authentication affecting cell function. Sci Rep 6. doi:10.1038/srep28994
Kurosawa, H. (2007). Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng 103, 389–398. doi:10.1263/jbb.103.389
Langdon, S. P. (2004). Cell culture contamination: an overview. Methods Mol Med 88, 309–317. doi:10.1385/1-59259-406-9:309
Lei, M., Liang, D., Yang, Y. et al. (2020). Long-read DNA sequencing fully characterized chromothripsis in a patient with Langer–Giedion syndrome and Cornelia de Lange syndrome-4. J Hum Genet 65, 667–674. doi:10.1038/s10038-020-0754-6
Lenz, M., Goetzke, R., Schenk, A. et al. (2015). Epigenetic biomarker to support classification into pluripotent and non-pluripotent cells. Sci Rep 5. doi:10.1038/srep08973
Li, F., Hu, J., Xie, K. et al. (2015). Authentication of experimental materials: A remedy for the reproducibility crisis? Genes Dis 2, 283. doi:10.1016/j.gendis.2015.07.001
Liu, G., David, B. T., Trawczynski, M. et al. (2020). Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev Reports 16, 3–32. doi:10.1007/s12015-019-09935-x
Liu, W. and Chen, G. (2014). Cryopreservation of human pluripotent stem cells in defined medium. Curr Protoc Stem Cell Biol 2014, 1c.17.1-1c.17.13. doi:10.1002/9780470151808.sc01c17s31
Loring, J., Schwartz, P. and Wesselschmidt, R. (2007). Human Stem Cell Manual. 2nd ed. San Siego, CA: Academic Press.
Lorsch, J. R., Collins, F. S. and Lippincott-Schwartz, J. (2014). Fixing problems with cell lines. Science (80- ) 346, 1452–1453. doi:10.1126/science.1259110
MacArthur, D. G., Manolio, T. A., Dimmock, D. P. et al. (2014). Guidelines for investigating causality of sequence variants in human disease. Nature 508, 469–476. doi:10.1038/nature13127
MacLeod, R. A. F., Dirks, W. G., Matsuo, Y. et al. (1999). Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer 83, 555–563. doi:10.1002/(SICI)1097-0215(19991112)83:4<555::AID-IJC19>3.0.CO;2-2
Maddah, M., Shoukat-Mumtaz, U., Nassirpour, S. et al. (2014). A System for Automated, Noninvasive, Morphology-Based Evaluation of Induced Pluripotent Stem Cell Cultures. J Lab Autom 19, 454–460. doi:10.1177/2211068214537258
Mayshar, Y., Ben-David, U., Lavon, N. et al. (2010). Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 7, 521–531. doi:10.1016/j.stem.2010.07.017
McNutt, M. (2014). Journals unite for reproducibility. Science (80- ) 346, 679. doi:10.1126/science.aaa1724
de Miguel, M. P., Fuentes-Julián, S. and Alcaina, Y. (2010). Pluripotent Stem Cells: Origin, Maintenance and Induction. Stem Cell Rev Reports 6, 633–649. doi:10.1007/s12015-010-9170-1
Miyakawa, T. (2020). No raw data, no science: Another possible source of the reproducibility crisis. Mol Brain 13. doi:10.1186/s13041-020-0552-2
Müller, F.-J. (2014). Assessment of human pluripotent stem cells with PluriTest. StemBook. doi:10.3824/stembook.1.84.1
Müller, F. J., Schuldt, B. M., Williams, R. et al. (2011). A bioinformatic assay for pluripotency in human cells. Nat Methods 8, 315–317. doi:10.1038/nmeth.1580
Munafò, M. R., Nosek, B. A., Bishop, D. V. M. et al. (2017). A manifesto for reproducible science. Nat Hum Behav 1, 1–9. doi:10.1038/s41562-016-0021
Nelson-Rees, W. A., Daniels, D. W. and Flandermeyer, R. R. (1981). Cross-contamination of cells in culture. Science (80- ) 212, 446–451. doi:10.1126/science.6451928
NIH (2015). NIH Workshop on Reproducibility in cell culture studies. Available at: https://videocast.nih.gov/watch_16876
Nikfarjam, L. and Farzaneh, P. (2012). Prevention and detection of mycoplasma contamination in cell culture. Cell J 13, 203–212.
Ntai, A., Baronchelli, S., La Spada, A. et al. (2017). A review of research-grade human induced pluripotent stem cells qualification and biobanking processes. Biopreserv Biobank 15, 384–392. doi:10.1089/bio.2016.0097
O’Shea, O., Steeg, R., Chapman, C. et al. (2020). Development and implementation of large-scale quality control for the European bank for induced Pluripotent Stem Cells. Stem Cell Res 45. doi:10.1016/j.scr.2020.101773
OECD (2018). Guidance Document on Good In Vitro Method Practices (GIVIMP). Paris: OECD Series on Testing and Assessment, No. 286, OECD Publishing. doi:10.1787/9789264304796-en
Pamies, D., Bal-Price, A., Chesné, C. et al. (2018). Advanced Good Cell Culture Practice for human primary, stem cell-derived and organoid models as well as microphysiological systems. ALTEX 35, 353–378. doi:10.14573/altex.1710081
Pamies, D., Bal-Price, A., Simeonov, A. et al. (2017). Good cell culture practice for stem cells & stem-cell-derived models. ALTEX 34, 95–132. doi:10.14573/altex.1607121
Pamies, D., Leist, M., Coecke, S. et al. (2020). Good Cell and Tissue Culture Practice 2.0 (GCCP 2.0) - Draft for stakeholder discussion and call for action. ALTEX 37, 490–492. doi:10.14573/altex.2007091
Pistollato, F., Bremer-Hoffmann, S., Healy, L. et al. (2012). Standardization of pluripotent stem cell cultures for toxicity testing. Expert Opin Drug Metab Toxicol 8, 239–257. doi:10.1517/17425255.2012.639763
Rohani, L., Johnson, A. A., Naghsh, P. et al. (2018). Concise Review: Molecular Cytogenetics and Quality Control: Clinical Guardians for Pluripotent Stem Cells. Stem Cells Transl Med 7, 867–875. doi:10.1002/sctm.18-0087
Rojas, A., Gonzalez, I. and Figueroa, H. (2008). Cell line cross-contamination in biomedical research: A call to prevent unawareness. Acta Pharmacol Sin 29, 877–880. doi:10.1111/j.1745-7254.2008.00809.x
Rottem, S. and Barile, M. F. (1993). Beware of mycoplasmas. Trends Biotechnol 11, 143–151. doi:10.1016/0167-7799(93)90089-R
Sarafian, R., Morato-Marques, M., Borsoi, J. et al. (2018). Monitoring cell line identity in collections of human induced pluripotent stem cells. Stem Cell Res 28, 66–70. doi:10.1016/j.scr.2018.01.030
Sathananthan, A. H. and Trounson, A. (2005). Human embryonic stem cells and their spontaneous differentation. Ital J Anat Embryol, 151–7.
Scudellari, M. (2016). A decade of : iPS Cells. Nature 534, 310–312. doi:10.1038/534310a
Shibamiya, A., Schulze, E., Krauß, D. et al. (2020). Cell Banking of hiPSCs: A Practical Guide to Cryopreservation and Quality Control in Basic Research. Curr Protoc Stem Cell Biol 55. doi:10.1002/cpsc.127
Sikkema-Raddatz, B., Castedo, S. and Te Meerman, G. J. (1997). Probability tables for exclusion of mosaicism in prenatal diagnosis. Prenat Diagn 17, 115–118. doi:10.1002/(SICI)1097-0223(199702)17:2<115::AID-PD37>3.0.CO;2-A
Stacey, G. N., Crook, J. M., Hei, D. et al. (2013). Banking human induced pluripotent stem cells: Lessons learned from embryonic stem cells? Cell Stem Cell 13, 385–388. doi:10.1016/j.stem.2013.09.007
Stacey, G. N., Masters, J. R. W., Hay, R. J. et al. (2000). Cell contamination leads to inaccurate data: We must take action now. Nature 403, 356. doi:10.1038/35000394
Sullivan, S., Stacey, G. N., Akazawa, C. et al. (2018). Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med 13. doi:10.2217/rme-2018-0095
Suter-Dick, L., Alves, P. M., Blaauboer, B. J. et al. (2015). Stem cell-derived systems in toxicology assessment. Stem Cells Dev 24, 1284–1296. doi:10.1089/scd.2014.0540
Taapken, S. M., Nisler, B. S., Newton, M. A. et al. (2011). Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat Biotechnol 29, 313–314. doi:10.1038/nbt.1835
Takahashi, K., Tanabe, K., Ohnuki, M. et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. doi:10.1016/j.cell.2007.11.019
Timenetsky, J., Santos, L. M., Buzinhani, M. et al. (2006). Detection of multiple mycoplasma infection in cell cultures by PCR. Brazilian J Med Biol Res 39, 907–914. doi:10.1590/S0100-879X2006000700009
Wagner, K. and Welch, D. (2010). Cryopreserving and recovering of human iPS cells using complete knockout serum replacement feeder-free medium. J Vis Exp, 41. doi:10.3791/2237
Wakui, T. (2017). Method for evaluation of human induced pluripotent stem cell quality using image analysis based on the biological morphology of cells. J Med Imaging 4, 1. doi:10.1117/1.jmi.4.4.044003
WHO (2013). Annex 3 Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks Replacement of Annex 1 of WHO Technical Report Series, No. 878. Available at: http://www.who.int/biologicals/vaccines/TRS_978_Annex_3.pdf [Accessed January 13, 2021].
Xu, H., Baroukh, C., Dannenfelser, R. et al. (2013). ESCAPE: Database for integrating high-content published data collected from human and mouse embryonic stem cells. Database 2013. doi:10.1093/database/bat045
Yaffe, M. P., Noggle, S. A. and Solomon, S. L. (2016). Raising the standards of stem cell line quality. Nat Cell Biol 18, 236–237. doi:10.1038/ncb3313
Yu, M., Selvaraj, S. K., Liang-Chu, M. M. Y. et al. (2015). A resource for cell line authentication, annotation and quality control. Nature 520, 307–311. doi:10.1038/nature14397
Zhang, S., Liang, F., Lei, C. et al. (2019). Long-read sequencing and haplotype linkage analysis enabled preimplantation genetic testing for patients carrying pathogenic inversions. J Med Genet 56, 741–749. doi:10.1136/jmedgenet-2018-105976