New approach methods (NAMs) supporting read-across: Two neurotoxicity AOP-based IATA case studies
Main Article Content
Abstract
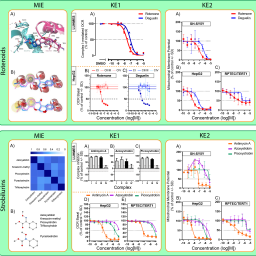
Read-across approaches are considered key in moving away from in vivo animal testing towards addressing data-gaps using new approach methods (NAMs). Ample successful examples are still required to substantiate this strategy. Here we present and discuss the learnings from two OECD IATA endorsed read-across case studies. They involve two classes of pesticides – rotenoids and strobilurins – each having a defined mode-of-action that is assessed for its neurological hazard by means of an AOP-based testing strategy coupled to toxicokinetic simulations of human tissue concentrations. The endpoint in question is potential mitochondrial respiratory chain mediated neurotoxicity, specifically through inhibition of complex I or III. An AOP linking inhibition of mitochondrial respiratory chain complex I to the degeneration of dopaminergic neurons formed the basis for both cases but was deployed in two different regulatory contexts. The two cases also exemplify several different read-across concepts: analogue versus category approach, consolidated versus putative AOP, positive versus negative prediction (i.e., neurotoxicity versus low potential for neurotoxicity), and structural versus biological similarity. We applied a range of NAMs to explore the toxicodynamic properties of the compounds, e.g., in silico docking as well as in vitro assays and readouts – including transcriptomics – in various cell systems, all anchored to the relevant AOPs. Interestingly, although some of the data addressing certain elements of the read-across were associated with high uncertainty, their impact on the overall read-across conclusion remained limited. Coupled to the elaborate regulatory review that the two cases underwent, we propose some generic learnings of AOP-based testing strategies supporting read-across.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Albrecht, W., Kappenberg, F., Brecklinghaus, T. et al. (2019). Prediction of human drug-induced liver injury (DILI) in relation to oral doses and blood concentrations. Archives of Toxicology, 93(6), 1609–1637. doi:10.1007/s00204-019-02492-9
Almeida, A., Almeida, J., Bolaños, J. P., and Moncada, S. (2001). Different responses of astrocytes and neurons to nitric oxide: The role of glycolytically generated ATP in astrocyte protection. Proceedings of the National Academy of Sciences of the United States of America, 98(26), 15294–15299. doi:10.1073/pnas.261560998
Almeida, A., Moncada, S., and Bolaños, J. P. (2004). Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nature Cell Biology, 6(1), 45. doi:10.1038/ncb1080
Ankley, G. T., Bennett, R. S., Erickson, R. J. et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. doi:10.1002/etc.34
Ball, N., Cronin, M. T. D., Shen, J. et al. (2016). Toward good read-across practice (GRAP) guidance. Altex. doi:10.14573/altex.1601251
Bartlett, D. W., Clough, J. M., Godwin, J. R. et al. (2002). The strobilurin fungicides. Pest Management Science. doi:10.1002/ps.520
Bennekou, S. H. (2019). Moving towards a holistic approach for human health risk assessment – Is the current approach fit for purpose? EFSA Journal. doi:10.2903/j.efsa.2019.e170711
Berezhkovskiy, L. M. (2004). Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. Journal of Pharmaceutical Sciences. doi:10.1002/jps.20073
Betarbet, R., Sherer, T. B., MacKenzie, G. et al. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience. doi:10.1038/81834
Busquet, F., Kleensang, A., Rovida, C. et al. (2020). New european union statistics on laboratory animal use - What really counts! Altex. doi:10.14573/altex.2003241
Caboni, P., Sherer, T. B., Zhang, N. et al. (2004). Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chemical Research in Toxicology. doi:10.1021/tx049867r
Chang, T. I., Horal, M., Jain, S. K. et al. (2003). Oxidant regulation of gene expression and neural tube development: Insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia. doi:10.1007/s00125-003-1063-2
Conboy, E., Selcen, D., Brodsky, M. et al. (2018). Novel Homozygous Variant in TTC19 Causing Mitochondrial Complex III Deficiency with Recurrent Stroke-Like Episodes: Expanding the Phenotype. Seminars in Pediatric Neurology. doi:10.1016/j.spen.2018.04.003
Delp, J., Cediel-Ulloa, A., Suciu, I. et al. (2021). Neurotoxicity and underlying cellular changes of 21 mitochondrial respiratory chain inhibitors. Archives of Toxicology. doi:10.1007/s00204-020-02970-5
Delp, J., Funke, M., Rudolf, F. et al. (2019). Development of a neurotoxicity assay that is tuned to detect mitochondrial toxicants. Archives of Toxicology. doi:10.1007/s00204-019-02473-y
Dhillon, A. S., Tarbutton, G. L., Levin, J. L. et al. (2008). Pesticide/environmental exposures and Parkinson’s disease in East Texas. Journal of Agromedicine. doi:10.1080/10599240801986215
Durant, J. L., Leland, B. A., Henry, D. R., and Nourse, J. G. (2002). Reoptimization of MDL keys for use in drug discovery. Journal of Chemical Information and Computer Sciences. doi:10.1021/ci010132r
Escher, S. E., Kamp, H., Bennekou, S. H. et al. (2019). Towards grouping concepts based on new approach methodologies in chemical hazard assessment: the read-across approach of the EU-ToxRisk project. Archives of Toxicology. doi:10.1007/s00204-019-02591-7
EU Scientific advice mechanism (2018) EU Authorisation Processes of Plant Protection Products – From a Scientific Point of View. Publications Office of the EU, Luxembourg. doi:10.2777/238919
European Parliament and the council (2009), Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC (OJ L 309, 24.11.2009, p. 1) http://data.europa.eu/eli/reg/2009/1107/oj
European Parliament and the council (2020) Evaluation of Regulation (EC) No 1107/2009 on the placing of plant protection products on the market and of Regulation (EC) No 396/2005 on maximum residue levels of pesticides. Brussels, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0208&from=EN
EC - European Commission (2019): EURL ECVAM dataset on alternative methods to animal experimentation (DB-ALM). PID: http://data.europa.eu/89h/b7597ada-148d-4560-9079-ab0a5539cad3
Fisher, C., Siméon, S., Jamei, M. et al. (2019). VIVD: Virtual in vitro distribution model for the mechanistic prediction of intracellular concentrations of chemicals in in vitro toxicity assays. Toxicology in vitro. doi:10.1016/j.tiv.2018.12.017
Ghezzi, D., Arzuffi, P., Zordan, M. et al. (2011). Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nature Genetics. doi:10.1038/ng.761
Herrero-Mendez, A., Almeida, A., Fernández, E. et al. (2009). The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nature Cell Biology, 11(6), 747–752. doi:10.1038/ncb1881
Krall, A. S., Mullen, P. J., Surjono, F. et al. (2021). Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabolism, 33, 1–14. doi:10.1016/j.cmet.2021.02.001
Krebs, A., Waldmann, T., Wilks, M. F. et al. (2019). Template for the description of cell-based toxicological test methods to allow evaluation and regulatory use of the data. Altex. doi:10.14573/altex.1909271
Kunii, M., Doi, H., Higashiyama, Y. et al. (2015). A Japanese case of cerebellar ataxia, spastic paraparesis and deep sensory impairment associated with a novel homozygous TTC19 mutation. Journal of Human Genetics. doi:10.1038/jhg.2015.7
Leach, A. R., and Gillet, V. J. (2007). An introduction to chemoinformatics. In An Introduction To Chemoinformatics. doi:10.1007/978-1-4020-6291-9s
Limonciel, A., Ates, G., Carta, G. et al. (2018). Comparison of base-line and chemical-induced transcriptomic responses in HepaRG and RPTEC/TERT1 cells using TempO-Seq. Archives of Toxicology. doi:10.1007/s00204-018-2256-2
Mav, D., Shah, R. R., Howard, B. E. et al. (2018). A hybrid gene selection approach to create the S1500+ targeted gene sets for use in high-throughput transcriptomics. PLoS ONE. doi:10.1371/journal.pone.0191105
Mordaunt, D. A., Jolley, A., Balasubramaniam, S. et al. (2015). Phenotypic variation of TTC19-deficient mitochondrial complex III deficiency: A case report and literature review. American Journal of Medical Genetics, Part A. doi:10.1002/ajmg.a.36968
Ockleyford, C. (2017). Investigation into experimental toxicological properties of plant protection products having a potential link to Parkinson’s disease and childhood leukaemia†. EFSA Journal. doi:10.2903/j.efsa.2017.4691
OECD - Organisation for Economic Co-operation and Development (1997), Test No. 424: Neurotoxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, doi:10.1787/9789264071025-en
OECD (2016). Guidance document for the use of adverse outcome pathways in developing integrated approaches to testing and assessment (IATA), Series on Testing & Assessment No.260, Paris, ENV/JM/MONO(2016)67, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)67&doclanguage=en
OECD (2020a). van der Stel, W., Bennekou, S. H., Carta, G., Eakins, J., Delp, J., Forsby, A., Kamp, H., Gardner, I., Zdradil, B., Pastor, M., Gomes, J. C., White, A., Steger-Hartmann, T., Danen, E. H. J., Leist, M., Walker, P., Jennings, P., & van de Water, B. Case study on the use of integrated approaches to testing and assessment for identification and characterisation of parkinsonian hazard liability of deguelin by an aop-based testing and read across approach: Series on Testing and Assessment No. 326, ENV/JM/MONO(2020)22 https://orbit.dtu.dk/en/publications/case-study-on-the-use-of-integrated-approaches-to-testing-and-ass-2
OECD (2020b). Bennekou, S. H., van der Stel, W., Carta, G., Eakins, J., Delp, J., Forsby, A., Kamp, H., Gardner, I., Zdradil, B., Pastor, M., Gomes, J. C., White, A., Steger-Hartmann, T., Danen, E. H. J., Leist, M., Walker, P., Jennings, P., & van de Water, B. Case study on the use of integrated approaches to testing and assessment for mitochondrial complex-iii-mediated neurotoxicity of azoxystrobin - read-across to other strobilurins: Series on testing and assessment no. 327, ENV/JM/MONO(2020)23 https://orbit.dtu.dk/en/publications/case-study-on-the-use-of-integrated-approaches-to-testing-and-ass
OECD (2020c), Report on considerations form case studies on integrated approaches for testing and assessment (IATA), Series on Testing & Assessment No.328, Paris, ENV/JM/MONO(2020)24 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)24&doclanguage=en
Pearson, B. L., Simon, J. M., McCoy, E. S. et al. (2016). Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nature Communications. doi:10.1038/ncomms11173
Poulin, P. and Theil, F. P. (2002). Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. Journal of Pharmaceutical Sciences. doi:10.1002/jps.10005
Rodgers, T. and Rowland, M. (2006). Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. Journal of Pharmaceutical Sciences. doi:10.1002/jps.20502
Sarewicz, M. and Osyczka, A. (2015). Electronic connection between the quinone and cytochrome c redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiological Reviews. doi:10.1152/physrev.00006.2014
SAPEA, Science Advice for Policy by European Academies. (2018) Improving authorisation processes for plant protection products in Europe: a scientific perspective on the potential risks to human health. Berlin: SAPEA. doi:10.26356/plantprotectionproducts
Schildknecht, S., Di Monte, D. A., Pape, R. et al. (2017). Tipping Points and Endogenous Determinants of Nigrostriatal Degeneration by MPTP. Trends in Pharmacological Sciences, Vol. 38, pp. 541–555. doi:10.1016/j.tips.2017.03.010
Schreyer, A. M. and Blundell, T. (2012). USRCAT: Real-time ultrafast shape recognition with pharmacophoric constraints. Journal of Cheminformatics. doi:10.1186/1758-2946-4-27
Sharma, L., Lu, J. and Bai, Y. (2009). Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Current Medicinal Chemistry. doi:10.2174/092986709787846578
Tanner, C. M., Kame, F., Ross, G. W. et al. (2011). Rotenone, paraquat, and Parkinson’s disease. Environmental Health Perspectives. doi:10.1289/ehp.1002839
Terron, A., Bal-Price, A., Paini, A. et al. (2018). An adverse outcome pathway for parkinsonian motor deficits associated with mitochondrial complex I inhibition. Archives of Toxicology. doi:10.1007/s00204-017-2133-4
Troger, F., Delp, J., Funke, M. et al. (2020). Identification of mitochondrial toxicants by combined in silico and in vitro studies – A structure-based view on the adverse outcome pathway. Computational Toxicology. doi:10.1016/j.comtox.2020.100123
Udeani, G. O., Zhao, G. M., Young Geun Shin, Y.et al. (2001). Pharmacokinetics of deguelin, a cancer chemopreventive agent in rats. Cancer Chemotherapy and Pharmacology. doi:10.1007/s002800000187
US-EPA, United States Environmental Protection Agency (2020). New approach methods work plan: Reducing use of animals in chemical testing. U.S. Environmental Protection Agency, Washington, DC. EPA 615B2001 https://www.epa.gov/sites/production/files/2020-06/documents/epa_nam_work_plan.pdf
Van der Stel, W., Carta, G., Eakins, J. et al. (2020). Multiparametric assessment of mitochondrial respiratory inhibition in HepG2 and RPTEC / TERT1 cells using a panel of mitochondrial targeting agrochemicals. Archives of Toxicology, (0123456789). doi:10.1007/s00204-020-02792-5
Villeneuve, D. L., Crump, D., Garcia-Reyero, N. et al. (2014). Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicological Sciences. doi:10.1093/toxsci/kfu199
von Jagow, G. and Link, T. A. (1986). Use of specific inhibitors on the mitochondrial bc1 complex. Methods in Enzymology. doi:10.1016/S0076-6879(86)26026-7
Waldmann, T., Rempel, E., Balmer, N. V. et al. (2014). Design principles of concentration-dependent transcriptome deviations in drug-exposed differentiating stem cells. Chemical Research in Toxicology. doi:10.1021/tx400402j
WHO - World Health Organization (2010) International Programme on Chemical Safety & Inter-Organization Programme for the Sound Management of Chemicals. Characterization and application of physiologically based phamacokinetic models in risk assessment. https://apps.who.int/iris/handle/10665/44495
Yeakley, J. M., Shepard, P. J., Goyena, D. E. et al. (2017). A Trichostatin a expression signature identified by TempO-Seq targeted whole transcriptome profiling. PLoS ONE. doi:10.1371/journal.pone.0178302