A cell-based alternative to the mouse potency assay for pharmaceutical type E botulinum antitoxins
Main Article Content
Abstract
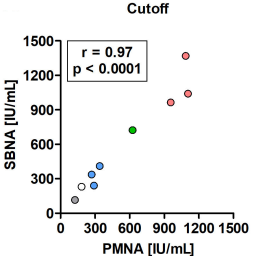
The pharmacopeia mouse neutralization assay (PMNA) is the standard method for determining the potency of pharmaceutical botulinum antitoxins. However, a PMNA requires a large number of mice, and, thus, an alternative in vitro method to replace it is needed. Herein, we developed an in vitro SiMa cell line-based neutralization assay (SBNA), compatible with a PMNA design, for therapeutic antitoxins against type E botulinum neurotoxin (BoNT/E). The SBNA measures the residual cellular activity of BoNT/E following antitoxin neutralization in the SiMa lysate using a specific quantitative sandwich ELISA for its cleaved cellular target protein SNAP-25. The potencies of different pharmaceutical antitoxin preparations were determined by applying two different quantification approaches: (1) a cutoff value, in accordance with the pharmacopeia concept, and (2) nonlinear regression of a standard curve generated by serial dilutions of a standard antitoxin. Both approaches achieved accurate potencies compared to the PMNA (average %RE of ~16%). Furthermore, the SBNA was able to determine in vitro, for the first time, the accurate neutralizing activity (%RE ≤ 20) of next-generation equine and rabbit therapeutic antitoxins. Collectively, a high correlation between SBNA and PMNA results was obtained for all antitoxin preparations (r = 0.99, P < 0.0001 for the standard curve approach, and r = 0.97, p < 0.0001 for the cutoff approach). In conclusion, the SBNA can potentially replace the PMNA and markedly reduce the need for laboratory animals for the approval of botulinum antitoxin preparations.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Arnon, S. S., Schechter, R., Inglesby, T. V. et al. (2001). Botulinum toxin as a biological weapon: Medical and public health management. JAMA 285, 1059-1070. doi:10.1001/jama.285.8.1059
Bak, N., Rajagopal, S., Stickings, P. et al. (2017). SiMa cells for a serotype specific and sensitive cell-based neutralization test for botulinum toxin A and E. Toxins (Basel) 9, 230. doi:10.3390/toxins9070230
Behrensdorf-Nicol, H. A., Wild, E., Bonifas, U. et al. (2018). In vitro potency determination of botulinum neurotoxin serotype A based on its receptor-binding and proteolytic characteristics. Toxicol In Vitro 53, 80-88. doi:10.1016/j.tiv.2018.07.008
Ben David, A., Diamant, E., Barnea, A. et al. (2013). The receptor binding domain of botulinum neurotoxin serotype A (BoNT/A) inhibits BoNT/A and BoNT/E intoxications in vivo. Clin Vaccine Immunol 20, 1266-1273. doi:10.1128/cvi.00268-13
Ben David, A., Torgeman, A., Barnea, A. et al. (2015). Expression, purification and characterization of the receptor-binding domain of botulinum neurotoxin serotype B as a vaccine candidate. Protein Expr Purif 110, 122-129. doi:10.1016/j.pep.2015.02.008
Binz, T., Blasi, J., Yamasaki, S. et al. (1994). Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269, 1617-1620. doi:10.1016/S0021-9258(17)42071-0
Bitz, S. (2010). The botulinum neurotoxin LD50 test – Problems and solutions. ALTEX 27, 114-116. doi:10.14573/altex.2010.2.114
Bowmer, E. J. (1963). Preparation and assay of the international standards for Clostridium botulinum types A, B, C, D and E antitoxins. Bull World Health Organ 29, 701-709.
Dembek, Z. F., Smith, L. A. and Rusnak, J. M. (2007). Botulism: Cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep 1, 122-134. doi:10.1097/DMP.0b013e318158c5fd
DeSilva, B., Smith, W., Weiner, R. et al. (2003). Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res 20, 1885-1900. doi:10.1023/b:pham.0000003390.51761.3d
Diamant, E., Lachmi, B. E., Keren, A. et al. (2014). Evaluating the synergistic neutralizing effect of anti-botulinum oligoclonal antibody preparations. PLoS One 9, e87089. doi:10.1371/journal.pone.0087089
Diamant, E., Pass, A., Rosen, O. et al. (2018). A novel rabbit spirometry model of type E botulism and its use for the evaluation of postsymptom antitoxin efficacy. Antimicrob Agents Chemother 62, e02379-17. doi:10.1128/AAC.02379-17
Dressler, D. and Dirnberger, G. (2001). Botulinum toxin antibody testing: Comparison between the immunoprecipitation assay and the mouse diaphragm assay. Eur Neurol 45, 257-260. doi:10.1159/000052139
EDQM – European Directorate for the Quality of Medicines and Healthcare (2019a). Botulinum Toxin Type A / Type B for Injection. European directorate for the quality of medicines and healthcare. Vol. 10. Strasbourg, France: European Pharmacopoeia.
EDQM (2019b). Botulinum Antitoxin. European directorate for the quality of medicines and healthcare. Vol. 10. Strasbourg, France: European Pharmacopoeia.
Falach, R., Sapoznikov, A., Alcalay, R. et al. (2018). Generation of highly efficient equine-derived antibodies for post-exposure treatment of ricin intoxications by vaccination with monomerized ricin. Toxins (Basel) 10, 466. doi:10.3390/toxins10110466
Fernandez-Salas, E., Wang, J., Molina, Y. et al. (2012). Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS One 7, e49516. doi:10.1371/journal.pone.0049516
Hall, Y. H., Chaddock, J. A., Moulsdale, H. J. et al. (2004). Novel application of an in vitro technique to the detection and quantification of botulinum neurotoxin antibodies. J Immunol Methods 288, 55-60. doi:10.1016/j.jim.2004.02.011
Hanna, P. A. and Jankovic, J. (1998). Mouse bioassay versus western blot assay for botulinum toxin antibodies: Correlation with clinical response. Neurology 50, 1624-1629. doi:10.1212/WNL.50.6.1624
ICH – International Council for Harmonisation (2019). ICH Guideline M10 on Bioanalytical Method Validation. https://www.Ema.Europa.Eu/en/documents/scientific-guideline/draft-ich-guideline-m10-bioanalytical-method-validation-step-2b_en.pdf
Jones, R. G., Corbel, M. J. and Sesardic, D. (2006). A review of WHO international standards for botulinum antitoxins. Biologicals 34, 223-226. doi:10.1016/j.biologicals.2005.11.009
Kiris, E., Kota, K. P., Burnett, J. C. et al. (2014). Recent developments in cell-based assays and stem cell technologies for botulinum neurotoxin research and drug discovery. Expert Rev Mol Diagn 14, 153-168. doi:10.1586/14737159.2014.867808
Lindsey, C. Y., Smith, L. A., West, M. W. et al. (2003). Evaluation of a botulinum fragment C-based ELISA for measuring the humoral immune response in primates. Biologicals 31, 17-24. doi:10.1016/S1045-1056(02)00074-X
Marini, P., MacLeod, R. A., Treuner, C. et al. (1999). SiMa, a new neuroblastoma cell line combining poor prognostic cytogenetic markers with high adrenergic differentiation. Cancer Genet Cytogenet 112, 161-164. doi:10.1016/s0165-4608(98)00269-6
McNutt, P., Celver, J., Hamilton, T. et al. (2011). Embryonic stem cell-derived neurons are a novel, highly sensitive tissue culture platform for botulinum research. Biochem Biophys Res Commun 405, 85-90. doi:10.1016/j.bbrc.2010.12.132
Mechaly, A., Alcalay, R., Noy-Porat, T. et al. (2018). Novel phage display-derived anti-abrin antibodies confer post-exposure protection against abrin intoxication. Toxins (Basel) 10, 80. doi:10.3390/toxins10020080
Mechaly, A., Elia, U., Alcalay, R. et al. (2019). Inhibition of francisella tularensis phagocytosis using a novel anti-LPS scFv antibody fragment. Sci Rep 9, 11418. doi:10.1038/s41598-019-47931-w
Mechaly, A., Diamant, E., Alcalay, R. et al. (2021). Isolation and characterization of a highly specific monoclonal antibody targeting the botulinum neurotoxin type E exposed SNAP-25 neoepitope. bioRxiv. doi:10.1101/2021.09.16.460610
Montal, M. (2010). Botulinum neurotoxin: A marvel of protein design. Ann Rev Biochem 79, 591-617. doi:10.1146/annurev.biochem.051908.125345
Nuss, J. E., Ruthel, G., Tressler, L. E. et al. (2010). Development of cell-based assays to measure botulinum neurotoxin serotype A activity using cleavage-sensitive antibodies. J Biomol Screen 15, 42-51. doi:10.1177/1087057109354779
Palace, J., Nairne, A., Hyman, N. et al. (1998). A radioimmuno-precipitation assay for antibodies to botulinum A. Neurology 50, 1463-1466. doi:10.1212/WNL.50.5.1463
Pathe-Neuschäfer-Rube, A., Neuschäfer-Rube, F., Genz, L. et al. (2015). Botulinum neurotoxin dose-dependently inhibits release of neurosecretory vesicle-targeted luciferase from neuronal cells. ALTEX 32, 297-306. doi:10.14573/altex.1503061
Pathe-Neuschäfer-Rube, A., Neuschäfer-Rube, F., Haas, G. et al. (2018). Cell-based reporter release assay to determine the potency of proteolytic bacterial neurotoxins. Toxins (Basel) 10, 360. doi:10.3390/toxins10090360
Pellett, S., Tepp, W. H., Clancy, C. M. et al. (2007). A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS Lett 581, 4803-4808. doi:10.1016/j.febslet.2007.08.078
Pellett, S., Tepp, W. H., Toth, S. I. et al. (2010). Comparison of the primary rat spinal cord cell (RSC) assay and the mouse bioassay for botulinum neurotoxin type A potency determination. J Pharmacol Toxicol Methods 61, 304-310. doi:10.1016/j.vascn.2010.01.003
Pellett, S., Du, Z. W., Pier, C. L. et al. (2011). Sensitive and quantitative detection of botulinum neurotoxin in neurons derived from mouse embryonic stem cells. Biochem Biophys Res Commun 404, 388-392. doi:10.1016/j.bbrc.2010.11.128
Pellett, S., Tepp, W. H., Johnson, E. A. et al. (2017). Assessment of ELISA as endpoint in neuronal cell-based assay for bont detection using hiPSC derived neurons. J Pharmacol Toxicol Methods 88, 1-6. doi:10.1016/j.vascn.2017.04.013
Pirazzini, M., Rossetto, O., Eleopra, R. et al. (2017). Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol Rev 69, 200-235. doi:10.1124/pr.116.012658
Rosen, O., Ozeri, E., Barnea, A. et al. (2016). Development of an innovative in vitro potency assay for anti-botulinum antitoxins. Toxins (Basel) 8, 276. doi:10.3390/toxins8100276
Rusnak, J. M. and Smith, L. A. (2009). Botulinum neurotoxin vaccines: Past history and recent developments. Hum Vaccin 5, 794-805. doi:10.4161/hv.9420
Rust, A., Doran, C., Hart, R. et al. (2017). A cell line for detection of botulinum neurotoxin type B. Front Pharmacol 8, 796. doi:10.3389/fphar.2017.00796
Schiavo, G., Santucci, A., Dasgupta, B. R. et al. (1993). Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct cooh-terminal peptide bonds. FEBS Lett 335, 99-103. doi:10.1016/0014-5793(93)80448-4
Schiavo, G., Matteoli, M. and Montecucco, C. (2000). Neurotoxins affecting neuroexocytosis. Physiol Rev 80, 717-766. doi:10.1152/physrev.2000.80.2.717
Smith, L. A. and Rusnak, J. M. (2007). Botulinum neurotoxin vaccines: Past, present, and future. Crit Rev Immunol 27, 303-318. doi:10.1615/CritRevImmunol.v27.i4.20
Tannenbaum, J. and Bennett, B. T. (2015). Russell and Burch’s 3Rs then and now: The need for clarity in definition and purpose. J Am Assoc Lab Anim Sci 54, 120-132.
Taylor, K., Gericke, C. and Alvarez, L. R. (2019). Botulinum toxin testing on animals is still a Europe-wide issue. ALTEX 36, 81-90. doi:10.14573/altex.1807101
Torgeman, A., Diamant, E., Levin, L. et al. (2017a). An in vitro cell-based potency assay for pharmaceutical type A botulinum antitoxins. Vaccine 35, 7213-7216. doi:10.1016/j.vaccine.2017.11.015
Torgeman, A., Mador, N., Dorozko, M. et al. (2017b). Efficacy of inactivation of viral contaminants in hyperimmune horse plasma against botulinum toxin by low pH alone and combined with pepsin digestion. Biologicals 48, 24-27. doi:10.1016/j.biologicals.2017.06.003
Torgeman, A., Schwartz, A., Diamant, E. et al. (2018). Studying the differential efficacy of postsymptom antitoxin treatment in type A versus type B botulism using a rabbit spirometry model. Dis Model Mech 11, dmm035089. doi:10.1242/dmm.035089
US FDA – U.S. Food and Drug Administration (2018). Bioanalytical Method Validation – Guidance for Industry. https://www.Fda.Gov/files/drugs/published/bioanalytical-method-validation-guidance-for-industry.pdf
Wang, J., Meng, J., Lawrence, G. W. et al. (2008). Novel chimeras of botulinum neurotoxins A and E unveil contributions from the binding, translocation, and protease domains to their functional characteristics. J Biol Chem 283, 16993-17002. doi:10.1074/jbc.M710442200
Whelan, S. M., Elmore, M. J., Bodsworth, N. J. et al. (1992). The complete amino acid sequence of the Clostridium botulinum type-E neurotoxin, derived by nucleotide-sequence analysis of the encoding gene. Eur J Biochem 204, 657-667. doi:10.1111/j.1432-1033.1992.tb16679.x
Wild, E., Bonifas, U., Klimek, J. et al. (2016). In vitro potency determination of botulinum neurotoxin B based on its receptor-binding and proteolytic characteristics. Toxicol In Vitro 34, 97-104. doi:10.1016/j.tiv.2016.03.011
Yadirgi, G., Stickings, P., Rajagopal, S. et al. (2017). Immuno-detection of cleaved SNAP-25 from differentiated mouse embryonic stem cells provides a sensitive assay for determination of botulinum a toxin and antitoxin potency. J Immunol Methods 451, 90-99. doi:10.1016/j.jim.2017.09.007
Zarebski, L. M., Vaughan, K., Sidney, J. et al. (2008). Analysis of epitope information related to Bacillus anthracis and Clostridium botulinum. Expert Rev Vaccines 7, 55-74. doi:10.1586/14760584.7.1.55