Amplifying the impact of kidney microphysiological systems: Predicting renal clearance using mechanistic modelling based on reconstructed drug secretion
Main Article Content
Abstract
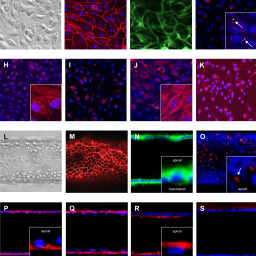
Accurate prediction of pharmacokinetic parameters, such as renal clearance, is fundamental to the development of effective and safe new treatments for patients. However, conventional renal models have a limited ability to predict renal drug secretion, a process that is dependent on transporters in the proximal tubule. Improvements in microphysiological systems (MPS) have extended our in vitro capabilities to predict pharmacokinetic parameters. In this study a kidney-MPS model was developed that successfully recreated renal drug secretion. Human proximal tubule cells grown in the kidney-MPS, resembling an in vivo phenotype, actively secreted the organic cation drug metformin and organic anion drug cidofovir, in contrast to cells cultured in conventional culture formats. Metformin and cidofovir renal secretory clearance were predicted from kidney-MPS data within 3.3- and 1.3-fold, respectively, of clinically reported values by employing a semi-mechanistic drug distribution model using kidney-MPS drug transport parameters together with in vitro to in vivo extrapolation. This approach introduces an effective application of a kidney-MPS model coupled with pharmacokinetic modelling tools to evaluate and predict renal drug clearance in humans. Kidney-MPS renal clearance predictions can potentially complement pharma-cokinetic animal studies and contribute to the reduction of pre-clinical species use during drug development.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Brater, D. C. (2002). Measurement of renal function during drug development. Br J Clin Pharmacol 54, 87-95. doi:10.1046/j.1365-2125.2002.01625.x
Brown, C. D. A., Sayer, R., Windass, A. S. et al. (2008). Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol 233, 428-438. doi:10.1016/j.taap.2008.09.018
Caetano-Pinto, P., Janssen, M. J., Gijzen, L. et al. (2016). Fluorescence-based transport assays revisited in a human renal proximal tubule cell line. Mol Pharm 13, 933-944. doi:10.1021/acs.molpharmaceut.5b00821
Caetano-Pinto, P. and Stahl, S. H. (2018). Perspective on the application of microphysiological systems to drug transporter studies. Drug Metab Dispos 46, 1647-1657. doi:10.1124/dmd.118.082750
Chapron, A., Chapron, B. D., Hailey, D. W. et al. (2020). An improved vascularized, dual-channel microphysiological system facilitates modeling of proximal tubular solute secretion. ACS Pharmacol Transl Sci 3, 496-508. doi:10.1021/acsptsci.9b00078
Cihlar, T., Lin, D. C., Pritchard, J. B. et al. (1999). The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol 56, 570-580. doi:10.1124/mol.56.3.570
Cirit, M. and Stokes, C. L. (2018). Maximizing the impact of microphysiological systems with: In vitro-in vivo translation. Lab Chip 18, 1831-1837. doi:10.1039/c8lc00039e
Cundy, K. C., Petty, B. G., Flaherty, J. et al. (1995). Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 39, 1247-1252. doi:10.1128/AAC.39.6.1247
Davies, B. and Morris, T. (1993). Physiological parameters in laboratory animals and humans. Pharm Res 10, 1093-1095. doi:10.1023/A:1018943613122
Davies, M., Jones, R. D. O., Grime, K. et al. (2020). Improving the accuracy of predicted human pharmacokinetics: Lessons learned from the AstraZeneca drug pipeline over two decades. Trends Pharmacol Sci 41, 390-408. doi:10.1016/j.tips.2020.03.004
Deutsch, B., Neumeister, C., Schwantes, U. et al. (2019). Interplay of the organic cation transporters OCT1 and OCT2 with the apically localized export protein MATE1 for the polarized transport of trospium. Mol Pharm 16, 510-517. doi:10.1021/acs.molpharmaceut.8b00779
Doi, K., Kimura, H., Matsunaga, Y. T. et al. (2022). Glomerulus-on-a-chip: Current insights and future potential towards recapitulating selectively permeable filtration systems. Int J Nephrol Renovasc Dis 15, 85-101. doi:10.2147/IJNRD.S344725
Duan, Y., Gotoh, N., Yan, Q. et al. (2008). Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci U S A 105, 11418-11423. doi:10.1073/pnas.0804954105
Dunn, C. J. and Peters, D. H. (1995). Metformin: A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs 49, 721-749. doi:10.2165/00003495-199549050-00007
Edington, C. D., Chen, W. L. K., Geishecker, E. et al. (2018). Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep 8, 4530. doi:10.1038/s41598-018-22749-0
Elsby, R., Chidlaw, S., Outteridge, S. et al. (2017). Mechanistic in vitro studies confirm that inhibition of the renal apical efflux transporter multidrug and toxin extrusion (MATE) 1, and not altered absorption, underlies the increased metformin exposure observed in clinical interactions with cimetidine, trimethoprim or pyrimethamine. Pharmacol Res Perspect 5, e00357. doi:10.1002/prp2.357
Ewart, L., Fabre, K., Chakilam, A. et al. (2017). Navigating tissue chips from development to dissemination: A pharmaceutical industry perspective. Exp Biol Med 242, 1579-1585. doi:10.1177/1535370217715441
Ewart, L. and Roth, A. (2021). Opportunities and challenges with microphysiological systems: A pharma end-user perspective. Nat Rev Drug Discov 20, 327-328. doi:10.1038/d41573-020-00030-2
Feng, B., LaPerle, J. L., Chang, G. et al. (2010). Renal clearance in drug discovery and development: Molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol 6, 939-952. doi:10.1517/17425255.2010.482930
Fukuda, Y., Kaishima, M., Ohnishi, T. et al. (2017). Fluid shear stress stimulates MATE2-K expression via Nrf2 pathway activation. Biochem Biophys Res Commun 484, 358-364. doi:10.1016/j.bbrc.2017.01.124
Graham, G. G., Punt, J., Arora, M. et al. (2011). Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50, 81-98. doi:10.2165/11534750-000000000-00000
Huang, W. and Isoherranen, N. (2018). Development of a dynamic physiologically based mechanistic kidney model to predict renal clearance. CPT Pharmacometrics Syst Pharmacol 7, 593-602. doi:10.1002/psp4.12321
Imaoka, T., Huang, W., Shum, S. et al. (2021). Bridging the gap between in silico and in vivo by modeling opioid disposition in a kidney proximal tubule microphysiological system. Sci Rep 11, 21356. doi:10.1038/s41598-021-00338-y
Isoherranen, N., Madabushi, R. and Huang, S. M. (2019). Emerging role of organ-on-a-chip technologies in quantitative clinical pharmacology evaluation. Clin Transl Sci 12, 113-121. doi:10.1111/cts.12627
Jang, K. J., Mehr, A. P., Hamilton, G. A. et al. (2013). Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5, 1119-1129. doi:10.1039/c3ib40049b
Jansen, J., De Napoli, I. E., Fedecostante, M. et al. (2015). Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci Rep 5, 16702. doi:10.1038/srep16702
Jansen, J., Fedecostante, M., Wilmer, M. J. et al. (2016). Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep 6, 26715. doi:10.1038/srep26715
Jansen, K., Pou Casellas, C., Groenink, L. et al. (2020). Humans are animals, but are animals human enough? A systematic review and meta-analysis on interspecies differences in renal drug clearance. Drug Discov Today 25, 706-717. doi:10.1016/j.drudis.2020.01.018
Jayagopal, A., Brakeman, P. R., Soler, P. et al. (2019). Apical shear stress enhanced organic cation transport in Human OCT2/MATE1-transfected madin-darby canine kidney cells involves ciliary sensing. J Pharmacol Exp Ther 369, 523-530. doi:10.1124/jpet.118.255026
Jenkinson, S. E., Chung, G. W., van Loon, E. et al. (2012). The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch 464, 601-611. doi:10.1007/s00424-012-1163-2
Kunze, A., Huwyler, J., Poller, B. et al. (2014). In vitro-in vivo extrapolation method to predict human renal clearance of drugs. J Pharm Sci 103, 994-1001. doi:10.1002/jps.23851
Lash, L. H., Putt, D. A. and Cai, H. (2006). Membrane transport function in primary cultures of human proximal tubular cells. Toxicology 228, 200-218. doi:10.1016/j.tox.2006.08.035
Liang, M., Ramsey, C. R. and Knox, F. G. (1999). The paracellular permeability of opossum kidney cells, a proximal tubule cell line. Kidney Int 56, 2304-2308. doi:10.1046/j.1523-1755.1999.00787.x
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408. doi:10.1006/meth.2001.1262
Maass, C., Stokes, C. L., Griffith, L. G. et al. (2017). Multi-functional scaling methodology for translational pharmacokinetic and pharmacodynamic applications using integrated microphysiological systems (MPS). Integr Biol 9, 290-302. doi:10.1039/c6ib00243a
Maass, C., Sorensen, N. B., Himmelfarb, J. et al. (2019). Translational assessment of drug-induced proximal tubule injury using a kidney microphysiological system. CPT Pharmacometrics Syst Pharmacol 8, 316-325. doi:10.1002/psp4.12400
Martovetsky, G., Tee, J. B. and Nigam, S. K. (2013). Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol 84, 808-823. doi:10.1124/mol.113.088229
Martovetsky, G., Bush, K. T. and Nigam, S. K. (2016). Kidney versus liver specification of SLC and ABC drug transporters, tight junction molecules, and biomarkers. Drug Metab Dispos 44, 1050-1060. doi:10.1124/dmd.115.068254
Mathialagan, S., Piotrowski, M. A., Tess, D. A. et al. (2017). Quantitative prediction of human renal clearance and drug-drug interactions of organic anion transporter substrates using in vitro transport data: A relative activity factor approach. Drug Metab Dispos 45, 409-417. doi:10.1124/dmd.116.074294
Maunsbach, A. B., Giebisch, G. H. and Stanton, B. A. (1987). Effects of flow rate on proximal tubule ultrastructure. Am J Physiol 253, F582-587. doi:10.1152/ajprenal.1987.253.3.f582
Morrissey, K. M., Wen, C. C., Johns, S. J. et al. (2012). The UCSF-FDA transportal: A public drug transporter database. Clin Pharmacol Ther 92, 545-546. doi:10.1038/clpt.2012.44
Moss, D. M., Neary, M. and Owen, A. (2014). The role of drug transporters in the kidney: Lessons from tenofovir. Front Pharmacol 5, 248. doi:10.3389/fphar.2014.00248
Motohashi, H. and Inui, K. I. (2013). Multidrug and toxin extrusion family SLC47: Physiological, pharmacokinetic and toxicokinetic importance of MATE1 and MATE2-K. Mol Aspects Med 34, 661-668. doi:10.1016/j.mam.2012.11.004
Neuhoff, S., Gaohua, L., Burt, H. et al. (2013). Accounting for transporters in renal clearance: Towards a mechanistic kidney model (Mech KiM). In Y. Sugiyama and B. Steffansen (eds), Transporters in Drug Development. AAPS Advances in the Pharmaceutical Sciences Series, Volume 7. New York, NY, USA: Springer. doi:10.1007/978-1-4614-8229-1_7
Nieskens, T. T. G., Persson, M., Kelly, E. J. et al. (2020). A multicompartment human kidney proximal tubule-on-a-chip replicates cell polarization-dependent cisplatin toxicity. Drug Metab Dispos 48, 1303-1311. doi:10.1124/DMD.120.000098
Nigam, S. K., Wu, W., Bush, K. T. et al. (2015). Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10, 2039-2049. doi:10.2215/CJN.02440314
Peel, S., Corrigan, A. M., Ehrhardt, B. et al. (2019). Introducing an automated high content confocal imaging approach for organs-on-chips. Lab Chip 19, 410-421. doi:10.1039/c8lc00829a
Petrosyan, A., Cravedi, P., Villani, V. et al. (2019). A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun 10, 3656. doi:10.1038/s41467-019-11577-z
Plosker, G. L. and Noble, S. (1999). Cidofovir. A review of its use in cytomegalovirus retinitis in patients with AIDS. Drugs 58, 325-345. doi:10.2165/00003495-199958020-00015
Ramsden, D. (2021). Leveraging microphysiological systems to address challenges encountered during development of oligonucleotide therapeutics. ALTEX 39, 273-296. doi:10.14573/altex.2108241
Redfern, W., Ewart, L., Hammond, T. et al. (2010). Impact and frequency of different toxicities throughout the pharmaceutical life cycle. J Pharmacol Toxicol Meth 62, e29. doi:10.1016/j.vascn.2010.11.098
Sakolish, C., Weber, E. J., Kelly, E. J. et al. (2018). Technology transfer of the microphysiological systems: A case study of the human proximal tubule tissue chip. Sci Rep 8, 14882. doi:10.1038/s41598-018-33099-2
Sakolish, C., Chen, Z., Dalaijamts, C. et al. (2020). Predicting tubular reabsorption with a human kidney proximal tubule tissue-on-a-chip and physiologically-based modeling. Toxicol In Vitro 63,104752. doi:10.1016/j.tiv.2019.104752
Scheen, A. J. (1996). Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30, 359-371. doi:10.2165/00003088-199630050-00003
Schindelin, J., Arganda-Carreras, I., Frise, E. et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat Methods 9, 676-682. doi:10.1038/nmeth.2019
Scotcher, D., Jones, C., Rostami-Hodjegan, A. et al. (2016). Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance. Eur J Pharm Sci 94, 59-71. doi:10.1016/j.ejps.2016.03.018
Shen, H., Liu, T., Jiang, H. et al. (2016). Cynomolgus monkey as a clinically relevant model to study transport involving renal organic cation transporters: In vitro and in vivo evaluation. Drug Metab Dispos 44, 238-249. doi:10.1124/dmd.115.066852
Sjögren, A. K., Breitholtz, K., Ahlberg, E. et al. (2018). A novel multi-parametric high content screening assay in ciPTEC-OAT1 to predict drug-induced nephrotoxicity during drug discovery. Arch Toxicol 92, 3175-3190. doi:10.1007/s00204-018-2284-y
Takeda, M., Narikawa, S., Hosoyamada, M. et al. (2001). Characterization of organic anion transport inhibitors using cells stably expressing human organic anion transporters. Eur J Pharmacol 419, 113-120. doi:10.1016/S0014-2999(01)00962-1
Van der Hauwaert, C., Savary, G., Buob, D. et al. (2014). Expression profiles of genes involved in xenobiotic metabolism and disposition in human renal tissues and renal cell models. Toxicol Appl Pharmacol 279, 409-418. doi:10.1016/j.taap.2014.07.007
Van der Made, T. K., Fedecostante, M., Scotcher, D. et al. (2019). Quantitative translation of microfluidic transporter in vitro data to in vivo reveals impaired albumin-facilitated indoxyl sulfate secretion in chronic kidney disease. Mol Pharm 16, 4551-4562. doi:10.1021/acs.molpharmaceut.9b00681
Van Meer, B. J., de Vries, H., Firth, K. S. A. et al. (2017). Small molecule absorption by PDMS in the context of drug response bioassays. Biochem Biophys Res Commun 482, 323-328. doi:10.1016/j.bbrc.2016.11.062
Van Ness, K. P., Chang, S. Y., Weber, E. J. et al. (2017). Microphysiological systems to assess nonclinical toxicity. Curr Protoc Toxicol 73, 14.18.1-14.18.28. doi:10.1002/cptx.27
Van Ness, K. P., Kelly, E. J. and Cesar, F. (2021). Microphysiological systems in adsorption, distribution, metabolism, and elimination sciences. Clin Transl Sci 15, 9-42. doi:10.1111/cts.13132
Vedula, E. M., Alonso, J. L., Arnaout, M. A. et al. (2017). A microfluidic renal proximal tubule with active reabsorptive function. PLoS One 12, e0184330. doi:10.1371/journal.pone.0184330
Verhulst, A., Sayer, R., De Broe, M. E. et al. (2008). Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol 74, 1084-1091. doi:10.1124/mol.108.047647
Vriend, J., Nieskens, T. T. G., Vormann, M. K. et al. (2018). Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip. AAPS J 20, 87. doi:10.1208/s12248-018-0247-0
Wang, H., Brown, P. C., Chow, E. C. Y. et al. (2021). 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin Transl Sci 14, 1659-1680. doi:10.1111/cts.13066
Wang, K., Sun, S., Li, L. et al. (2014). Involvement of organic cation transporter 2 inhibition in potential mechanisms of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 53, 90-98. doi:10.1016/j.pnpbp.2014.03.005
Weber, E. J., Chapron, A., Chapron, B. D. et al. (2016). Development of a microphysiological model of human kidney proximal tubule function. Kidney Int 90, 627-637. doi:10.1016/j.kint.2016.06.011
Yin, J. and Wang, J. (2016). Renal drug transporters and their significance in drug-drug interactions. Acta Pharm Sin B 6, 363-373. doi:10.1016/j.apsb.2016.07.013
Zamek-Gliszczynski, M. J., Taub, M. E., Chothe, P. P. et al. (2018). Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther 104, 890-899. doi:10.1002/cpt.1112