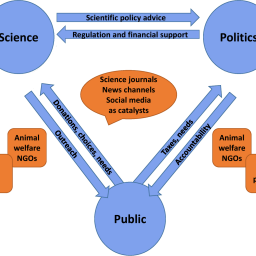
Engagement of scientists with the public and policymakers to promote alternative methods
Main Article Content
Abstract
Scientists are usually good at teaching, sometimes even to lay audiences. But communicating with journalists, activists, or policymakers can be a different story – hesitancy to make mistakes as well as the temptation to disproportionally promote one’s own case come into play. The multitude of social media and other web-based outlets has diversified and accelerated the communication of science. Real-time reactions, sharing of data, tools and results, increasing invitation of personal opinion, demand for transparency, political correctness, and loss of trust in experts are challenges to researchers in general. The field of alternatives to animal testing is more political and important to lay audiences than many others, so its scientists must be especially aware of these challenges. Public engagement offers the opportunity to form community and create wide support for non-animal research and its implementation. This requires scientists to step out of the ivory tower of higher education and engage with diverse interest groups by outreach activities, interviews, and press releases, etc. by employing tailored communication.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Avraamidou, L. and Osborne, J. (2009). The role of narrative in communicating science. Int J Sci Ed 31, 1683-1707, doi:10.1080/09500690802380695
Bao, L., Deng, W., Huang, B. et al. (2020). The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830-833. doi:10.1038/s41586-020-2312-y
Bertele, K. Feiereisen, S., Storey, C. et al. (2019). It’s not what you say, it’s the way you say it! Effective message styles for promoting innovative new services. J Bus Res 107, 38-49. doi:10.1016/j.jbusres.2019.09.024
Bottini, A. A. and Hartung, T. (2009). Food for thought … on economics of animal testing. ALTEX 26, 3-16. doi:10.14573/altex.2009.1.3
Bottini, A. A. and Hartung, T. (2010). The economics of animal testing. ALTEX 27, Spec Issue 1, 67-77. https://www.altex.org/index.php/altex/article/view/2214/2153
Busquet, F., Palopoli, M. and Hartung, T. (2014). Regulatory toxicology – Progress in law. In W. Town and J. N. Currano (eds), Science and the Law: Analytical Data in Support of Regulation in Health, Food, and the Environment (51-69, Volume 1147). Amer Chem Soc.
Busquet, F. and Hartung, T. (2017). The need for strategic development of safety sciences. ALTEX 34, 3-21. doi:10.14573/altex.1701031
Busquet, F. and Vinken, M. (2019). The use of social media in scientific research and creative thinking. Toxicol In Vitro 59, 51-54. doi:10.1016/j.tiv.2019.04.006
Busquet, F., Kleensang, A., Rovida, C. et al. (2020). New European Union statistics on laboratory animal use – What really counts! ALTEX 37, 167-186. doi:10.14573/altex.2003241
Canals, J., Romania, P., Belio-Mairal, P. et al. (2022). Advanced Non-animal Models in Biomedical Research. EUR 30334/4 EN, Publications Office of the European Union, Luxembourg, ISBN 978-92-76-49092-0. doi:10.2760/153339
Cassotta, M., Bartnicka, J. J., Pistollato, F. et al. (2022). A worldwide survey on the use of animal-derived materials and reagents in scientific experimentation. Eng Life Sci 22, 564-583. doi:10.1002/elsc.202100167
Celi, S., Cioffi, M., Capellini, K. et al. (2022). Advanced Non-animal Models in Biomedical Research. EUR 30334/5 EN, Publications Office of the European Union, Luxembourg. doi:10.2760/276664
Corbett, K. S., Edwards, D. K., Leist, S. R. et al. (2020). SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567-571. doi:10.1038/s41586-020-2622-0
Daneshian, M., Busquet, F., Hartung, T. et al. (2015). Animal use for science in Europe. ALTEX 32, 261-274. doi:10.14573/altex.1509081
EC – European Commission, Directorate-General for Research and Innovation, Group of Chief Scientific Advisors (2019). Scientific Advice to European Policy in a Complex World. Publications Office of the European Union. doi:10.2777/80320
EC – European Commission, Joint Research Centre, Holloway, M., Berggren, E. et al. (2021). Introducing the Three Rs into Secondary Schools, Universities and Continuing Education Programmes. Publications Office of the European Union. doi:10.2760/4378
Edler, J., Karaulova, M. and Barker, K. (2022). Understanding conceptual impact of scientific knowledge on policy: The role of policymaking conditions. Minerva 60, 209-233. doi:10.1007/s11024-022-09459-8
EU – European Union (2010). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJ L 276, 33-79. http://data.europa.eu/eli/dir/2010/63/oj
Fitzpatrick, B. G., Koustova, E. and Wang, Y. (2018). Getting personal with the “reproducibility crisis”: Interviews in the animal research community. Lab Anim 47, 175-177. doi:10.1038/s41684-018-0088-6
Folgiero, V., Romania, P., Rossi, F. et al. (2020). Advanced Non-animal Models in Biomedical Research. EUR 30334/1 EN, Publications Office of the European Union, Luxembourg. doi:10.2760/180550
Frank, J. (2005). Technological lock-in, positive institutional feedback, and research on laboratory animals. Struct Change Econ D 16, 557-575. doi:10.1016/j.strueco.2004.11.001
Gilbert N. (2010). Crucial data on REACH not disclosed. Nature 464, 1116-1117. doi:10.1038/4641116a
Gluck, J. (2019). Evidence over interests. In K. Herrmann and K. Jayne (eds), Animal Experimentation: Working Towards a Paradigm Change (689-691). Leiden, The Netherlands: Brill. https://www.jstor.org/stable/10.1163/j.ctvjhzq0f
Goldberg, A. (2015). A history of the Johns Hopkins Center for Alternatives to Animal Testing (CAAT): The first 28 years (1981-2009). Appl In Vitro Toxicol 1, 99-108. doi:10.1089/aivt.2015.0015
Gribaldo, L., Dura, A., Straccia, M. et al. (2021). Advanced Non-Animal Models in Biomedical Research – Immuno-oncology – Executive Summary. EUR 30334/3 EN, Publications Office of the European Union, Luxembourg. doi:10.2760/82873
Gribaldo, L. and Dura, A. (2022). EURL ECVAM literature review series on advanced non-animal models for respiratory diseases, breast cancer and neurodegenerative disorders. Animals 12, 2180. doi:10.3390/ani12172180
Griffin, G. and Locke, P. (2016). Comparison of the Canadian and US laws, regulations, policies, and systems of oversight for animals in research. ILAR J 57, 271-284. doi:10.1093/ilar/ilw037
Gruber, F. P. and Hartung, T. (2004). Alternatives to animal experimentation in basic research. ALTEX 21, Suppl 1, 3-31. doi:10.14573/altex.2004.suppl.3
Gruber, F. P. (2011). The Basel declaration: A critical appraisal. ALTEX 28, 353-354. doi:10.14573/altex.2011.4.353
Hartung, T. (2009). Food for thought … on evidence-based toxicology. ALTEX 26, 75-82. doi:10.14573/altex.2009.2.75
Hartung, T. and Rovida, C. (2009). Chemical regulators have overreached. Nature 460, 1080-1081. doi:10.1038/4601080a
Hartung, T. (2013). Look back in anger – What clinical studies tell us about preclinical work. ALTEX 30, 275-291. doi:10.14573/altex.2013.3.275
Hartung, T. (2021). Evidence integration in the era of information flooding – The advent of the comprehensive review. Front Public Health 9, 763828. doi:10.3389/fpubh.2021.763828
Hartung, T. and Tsatsakis, A. M. (2021). The state of the scientific revolution in toxicology. ALTEX 38, 379-386. doi:10.14573/altex.2106101
Herrmann, K. and Jayne, K. (eds) (2019). Animal Experimentation: Working Towards a Paradigm Change. Leiden, The Netherlands: Brill. https://www.jstor.org/stable/10.1163/j.ctvjhzq0f
Hynes, J., Marshall, L., Adcock, I. et al. (2020). Advanced Non-animal Models in Biomedical Research. EUR 30334 EN, Publications Office of the European Union, Luxembourg. ISBN 978-92-76-21381-9, JRC118161. doi:10.2760/52671
Kang, I., Smirnova, L., Kuhn, J. H. et al. (2021). COVID-19 – Prime time for microphysiological systems, as illustrated for the brain. ALTEX 38, 535-549. doi:10.14573/altex.2110131
Kazatchkine, M., Kinderlerer, J. and Gilligan, A. (2017). Brussels declaration: Twenty-point plan for science policy. Nature 541, 289. doi:10.1038/541289a
Knight, J., Rovida, C., Kreiling, R. et al. (2021). Continuing animal tests on cosmetic ingredients for REACH in the EU. ALTEX 38, 653-668. doi:10.14573/altex.2104221
Leist, M., Hasiwa, N., Rovida, C. et al. (2014). Consensus report on the future of animal-free systemic toxicity testing. ALTEX 31, 341-356. doi:10.14573/altex.1406091
Locke, P. A. and Myers, D. B. J. (2011). A replacement-first approach to toxicity testing is necessary to successfully reauthorize TSCA. ALTEX 28, 266-272. doi:10.14573/altex.2011.4.266
Locke, P. A. (2016). Ten fundamental legal terms and concepts that are useful in understanding laboratory animal law across nations. ILAR J 57, 266-270. doi:10.1093/ilar/ilw036
Locke, P., Singer, M. and Hartung, T. (2021). The humane research and testing act: Advancing science by creating a new center for alternatives at the US national institutes of health. ALTEX 38, 678-680. doi:10.14573/altex.2106031
Luechtefeld, T., Maertens, A., Russo, D. P. et al. (2016). Global analysis of publicly available safety data for 9,801 substances registered under REACH from 2008-2014. ALTEX 33, 95-109. doi:10.14573/altex.1510052
Marty, M. S., Andrus, A. K. and Groff, K. A. (2022). Animal metrics: Tracking contributions of new approach methods to reduced animal use. ALTEX 39, 95-112. doi:10.14573/altex.2107211
McCarthy, J., Herrmann, K., Sullivan, K. et al. (2021). Summer school on innovative approaches in science. ALTEX 38, 158-162. doi:10.14573/altex.2012153
Meigs, L., Smirnova, L., Rovida, C. et al. (2018). Animal testing and its alternatives – The most important omics is economics. ALTEX 35, 275-305. doi:10.14573/altex.1807041
Murphy, C., Naderlinger, E., Mater, A. et al. (2022). Comparison of human recombinant protein coatings and fibroblast-ECM to Matrigel for induced pluripotent stem cell culture and renal podocyte differentiation. ALTEX, online ahead of print. doi:10.14573/altex.2112204
Neuhaus, W. (2021). Consensus statement from the European Network of 3R Centres (EU3Rnet). ALTEX 38, 138-139. doi:10.14573/altex.2010061
Nogrady, B. (2021). ‘I hope you die’: How the COVID pandemic unleashed attacks on scientists. Nature 598, 250-253. doi:10.1038/d41586-021-02741-x
Pallocca, G., Rovida, C. and Leist, M. (2022a). On the usefulness of animals as a model system (part I): Overview of criteria and focus on robustness. ALTEX 39, 347-353. doi:10.14573/altex.2203291
Pallocca, G. and Leist, M. (2022b). On the usefulness of animals as a model system (part II): Considering benefits within distinct use domains. ALTEX 39, 531-539. doi:10.14573/altex.2207111
Pielke, Jr., R. (2007). The Honest Broker: Making Sense of Science in Policy and Politics. Cambridge, UK: Cambridge University Press. doi:10.1017/CBO9780511818110
Pound, P. and Ram, R. (2020). Are researchers moving away from animal models as a result of poor clinical translation in the field of stroke? An analysis of opinion papers. BMJ Open Sci 4, e100041. doi:10.1136/bmjos-2019-100041
Pronker, E. S., Weenen, T. C., Commandeur, H. et al. (2013). Risk in vaccine research and development quantified. PLoS One 8, e57755. doi:10.1371/journal.pone.0057755
Ritskes-Hoitinga, M. (2022). Medical regulators: Look beyond animal tests – Flexible approaches used to accelerate COVID-19 vaccines deserve wider uptake. Nature 604, 599. doi:10.1038/d41586-022-01110-6
Romania, P., Folgiero, V., Nic, M. et al. (2021). Advanced Non-animal Models in Biomedical Research. EUR 30334/3 EN, Publications Office of the European Union, Luxembourg. doi:10.2760/412567
Rovida C. and Hartung T. (2009). Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements. ALTEX 26,187-208. doi:10.14573/altex.2009.3.187
Schwedhelm, P., Kusnick, J., Heinl, C. et al. (2021). How many animals are used for SARS-CoV-2 research? An overview on animal experimentation in pre-clinical and basic research. EMBO Rep 22, e53751. doi:10.15252/embr.202153751
Topp, L., Mair, D., Smillie, L. et al. (2018). Knowledge management for policy impact: The case of the European Commission’s Joint Research Centre. Palgrave Commun 4, 87. doi:10.1057/s41599-018-0143-3
van der Valk, J., Bieback, K., Buta, C. et al. (2018). Fetal bovine serum (FBS): Past – present – future. ALTEX 35, 99-118. doi:10.14573/altex.1705101
Witters, H., Verstraelen, S., Aerts, L. et al. (2021). Advanced Non-animal Models in Biomedical Research. EUR 30334/2 EN, Publications Office of the European Union, Luxembourg. doi:10.2760/093006