Recommendations to improve the EU non-technical summaries of animal experiments
Main Article Content
Abstract
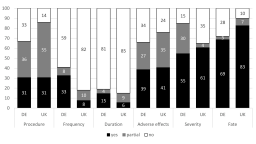
Under the new Directive 2010/63/EU, member states have to publish non-technical summaries (NTS) of the projects involving animals that they authorise. These summaries must include information on the objectives of the project including the predicted harm and benefits and the number and types of animals to be used. Summaries should also demonstrate compliance with the 3Rs. The intention was that NTS would help increase the transparency of animal research in the EU. In this article, we review the status of the publication of NTS across member states and give some general observations on publication speed, identification, accessibility and quality. We also review in more detail the quality of reporting in a selection of NTS from Germany and the UK. We consistently found that NTS from Germany and the UK were deficient in their description of what is being done to the animals and what they might experience as a result. Using examples taken from specific NTS we highlight what we view to be good and bad examples to assist member states and researchers in producing better NTS in the future. The NTS can also be an important tool in sharing of best practice in the 3Rs and the avoidance of duplicative animal testing. For this to happen, however, member states need to publish timely, ensure that NTS are accurate and, ideally, there needs to be some centralisation of the NTS.
Article Details
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).