Dear readers,
this issue starts with congratulations on the recent 80th birthday of our colleague and friend, Franz P. Gruber, and a tribute to his achievements in the field of alternatives.
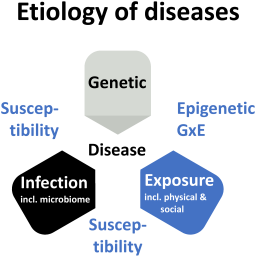
In his Food for Thought … contribution, Thomas Hartung calls for a Human Exposome Project. He argues that the technologies needed to achieve this vision of understanding how exposure, next to genetics and pathogens, impacts human health and causes disease are available and can be combined with artificial intelligence to integrate evidence in a way we could only dream of a short time ago.
Alex Cayley et al. describe a network of carcinogenicity adverse outcome pathways (AOPs) derived from the predictive system Derek Nexus by associating 37 AOPs with 60 assays, 351 in silico alerts, and data on 13,400 compounds. The authors explore different ways the network can be used and questioned to inform carcinogenicity safety assessments.
The GARD™skin assay was included in OECD TG 442E in 2022 as the first method employing expression of a defined set of genes to predict sensitization potential. Andy Forreryd and colleagues successfully challenge the assay with chemicals that are considered difficult to test, i.e., haptens, hydrophobic substances, and substances of unknown or variable composition, complex reaction products or biological substances (UVCBs).
While the rat is commonly used to assess endocrine disrupting potential, there is uncertainty about how well it predicts such effects in humans. In back-to-back publications, Diana Karwelat and Julia Kuehnlenz and colleagues describe a rat and a human thyroid-liver chip for the assessment of both direct and metabolism-dependent thyroid perturbation by environmental chemicals based on two endpoints: modulation of thyroid hormone secretion and its metabolism. They envisage that the models can be used to investigate species-specific differences, to allow quantitative in vitro to in vivo extrapolation, and thus to reduce and replace in vivo testing.
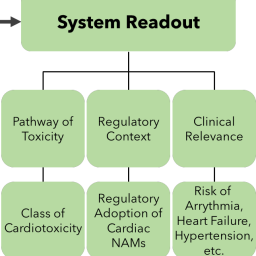
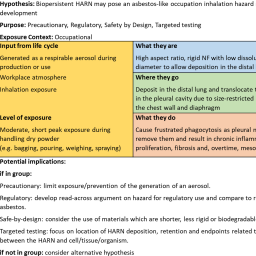
While new approach methodologies (NAMs) to assess the potential cardiotoxicity of pharmaceuticals are well underway, they have not yet been applied to environmental chemicals. Mark Daley et al. explore how these NAMs can be transferred and adapted to the diversity of these chemicals and what biological and regulatory considerations need to be considered in this endeavor.
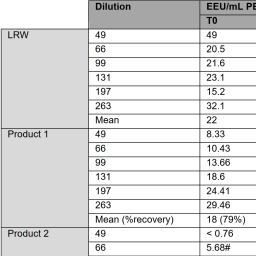
“Low endotoxin recovery” describes the phenomenon that some pharmaceutical products mask contaminating endotoxins from detection by pyrogen tests, leading to such alerts in bacterial endotoxin tests (BET) triggering confirmation in the rabbit pyrogen test (RPT). Tammy Thurman and colleagues compare endotoxin spike recovery in water or product samples using the BET, RPT, and monocyte-activation test (MAT). They find that the endotoxin spike is masked by some products in all three tests, indicating biological inactivation. Further, they confirm that the BET and MAT detect endotoxin spikes in water with a higher sensitivity than the RPT.
Fiona Murphy and colleagues share the concept developed within the GRACIOUS Framework to group nanoforms for risk assessment using a structured approach. The presented template gathers the relevant information and generates a grouping hypothesis by an integrated approach to testing and assessment (IATA). This approach can improve the efficiency and throughput of risk assessment of nanoforms.
Initiatives to replace fetal bovine serum have also drawn attention to other animal-derived products used in cell culture, especially Matrigel, which is derived from sarcomas grown in mice. Cormac Murphy and colleagues find that different recombinant protein coatings and extracellular matrix derived from fibroblasts can substitute for Matrigel for the maintenance culture of different induced pluripotent stem cell lines (iPSCs) and allow their differentiation to renal podocytes.
In their BenchMarks contribution, Elijah Petersen et al. describe a framework for the quality assessment of a new approach methodology and give examples of artifacts and biases revealed by quality control measurements. The set of tools can help to optimize test reproducibility.
Our events calendar for 2023 is already packed with webinars and meetings, especially the 2nd MPS World Summit in Berlin, Germany and the 12th World Congress on Alternatives and Animal Use in the Life Sciences in Niagara Falls, Canada. Both are open for abstract submissions!
With best wishes for a healthy and successful 2023,
Sonja von Aulock
Editor-in-chief
Congratulations, Franz P. Gruber!
On November 27, we celebrated the 80th birthday of our friend, colleague, and mentor Franz Paul Gruber, a tireless advocate for alternatives to animal testing, whose impressive career has been fully dedicated to serving the best interests of animals. Franz is an iconic figure in the field of alternative methods, who has worked long and hard to earn the field the recognition it deserves.
Franz realized early on in his career how difficult the position of animals is in our society. He studied veterinary medicine and practiced as a veterinarian for a couple of years. After earning his doctorate, he accepted a position as a research associate and assistant professor at the department of laboratory animal sciences at the Free University in Berlin, where he qualified as an expert veterinarian and earned a habilitation degree. In 1977 he moved to the University of Konstanz to plan and set up the animal research facility as its academic director. A revolutionary principle of this time was the inclusion of cell culture laboratories to produce antibodies by animal-free methods.
Franz’ interest in the 3Rs (replace, reduce, and refine) started in the early 1980s. Besides being active as an animal welfare officer and a member of the animal welfare commission of the regional council of Freiburg, he started to work for the nascent journal ALTEX in 1986 and joined the scientific board of the Foundation SET in 1987. In 1993, he decided to focus entirely on alternatives to animal experimentation when he moved to Zurich and accepted the position of editor-in-chief of ALTEX and scientific advisor of Animalfree Research (then FFVFF). During his time as editor-in-chief of ALTEX until 2010, Franz professionalized the journal and worked tirelessly to grow its recognition. What started rather like an educational leaflet written in German grew into one of the most influential, open-access journals in the field. Franz has been the president of the Doerenkamp-Zbinden Foundation for animalfree research since 2005, and in 2006 he established ALTEX Edition, the society that publishes the journals ALTEX, ALTEX Proceedings, and TIERethik, and functioned as its CEO until 2018.
Franz is an inspiring figure who has showed us how, with patience, dedication, integrity, and passion for a good cause, to make a positive change and move things forward. He personally introduced some of us to the world of alternative methods and guided our path into this community. His lecture series on alternative methods provided background and opportunities for young post-docs to join in and teach about their research on alternatives. It has always been important for him not only to grow his own knowledge and experience but also to share them with others. Thomas Hartung often recalls how he first shared his idea for a human pyrogen test with Franz the same evening over a beer, and he replied, “Write this up for ALTEX!” His principle of sharing and spreading the gospel was implemented formally by the establishment of professorships to teach the young generation about alternative methods. Franz’ greatest success is the implementation of the 3Rs in academic curricula of renowned universities around the world. This was achieved by the Doerenkamp Zbinden Foundation sponsoring endowed chairs at Johns Hopkins University (Baltimore, US), the University of Utrecht (NL), the University of Konstanz (D), in Chennai/Tiruchirapalli (India), and at the University of Geneva (CH). For several of these professors this was instrumental in allowing them to build a career on new approach methods. Franz accompanied and enabled their continuous growth over two decades and extends this support until today.
For several decades, Franz has represented both evidence-based science and animal welfare, allowing both “worlds” not only to profit from one another but to grow together. The 3Rs field would never be what it is now without this determined pioneer advocating for better, animalfree science. “Retirement does not exist in my dictionary,” he said once. He is still fully engaged in promoting alternatives to animal testing as the president of the Doerenkamp-Zbinden Foundation for animalfree research, the president of ALTEX Edition, the co-president and board member of the society Doctors for Animal Protection in Medicine, and member of the scientific committee of EUSAAT.
Thank you, our dear Franz, for being such an inspiring and dedicated colleague and a genuine friend to many of us. It is a true privilege to work with you. We wish you all the best and look forward to enjoying your company and collaborating with you for many more years to come. Together we are stronger and every day closer to reaching our common goals!
Your colleagues at ALTEX Edition and the Doerenkamp Zbinden Foundation
A call for a Human Exposome Project
https://doi.org/10.14573/altex.2301061
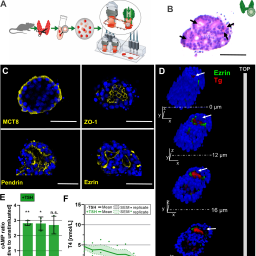
A microfluidic thyroid-liver platform to assess chemical safety in humans
https://doi.org/10.14573/altex.2108261
A rodent thyroid-liver chip to capture thyroid toxicity on organ function level
https://doi.org/10.14573/altex.2108262
Comparison of pyrogen assays by testing products exhibiting low endotoxin recovery
https://doi.org/10.14573/altex.2202021
How to formulate hypotheses and IATAs to support grouping and read-across of nanoforms
https://doi.org/10.14573/altex.2203241
CellTox Days 2022 – Inside the barriers: In vitro models and their applications
https://doi.org/10.14573/altex.2211101
Technical framework for enabling high quality measurements in new approach methodologies (NAMs)
https://doi.org/10.14573/altex.2205081