Performance of monocyte activation test supplemented with human serum compared to fetal bovine serum
Main Article Content
Abstract
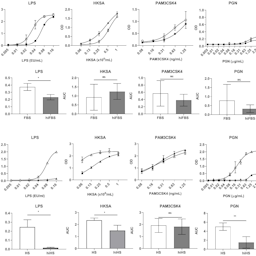
The monocyte activation test (MAT) is used to detect pyrogens in pharmaceutical products and serves as replacement of the rabbit pyrogen test. The peripheral blood mononuclear cell-based MAT assay requires the addition of serum to the medium and is performed with either fetal bovine serum (FBS) or human serum (HS). Since the capacity to detect non-endotoxin pyrogens (NEPs) in a sensitive manner is an important strength of MAT compared to the bacterial endotoxin test, the performance of the MAT using FBS and HS was compared using endotoxin and several NEPs. The MAT was more sensitive for endotoxin when FBS was used, however for most NEPs the MAT was more sensitive when performed in HS. Furthermore, heat-inactivation of FBS affected the performance of the MAT for endotoxin to some extent but not for the NEPs. Interestingly, heat-inactivation of HS led to an almost complete loss of reactivity towards endotoxin, reduced the response towards heat-killed Staphylococcus aureus and peptidoglycan, but had minor or no effects on the responses towards R848, flagellin, and Pam3CSK4. Product testing of a human blood-derived product in MAT using HS was beneficial since endotoxin spike recoveries were improved. This product is therefore currently batch released with the HS-based MAT assay. Overall, to guarantee optimal performance of MAT, heat-inactivated serum should be avoided. The HS-based MAT appears to be the first choice to replace the rabbit pyrogen test, while in some cases the FBS-based MAT may be favored.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Berbee, J. F., Havekes, L. M. and Rensen, P. C. (2005). Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res 11, 97-103. doi:10.1179/096805105X35215
Copeland, S., Warren, H. S., Lowry, S. F. et al. (2005). Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12, 60-67. doi:10.1128/CDLI.12.1.60-67.2005
EDQM – European Directorate for the Quality of Medicines and HealthCare (2020). Monocyte Activation Test Chapter 2.6.30 European Pharmacopoeia (Version 07/2017), 10th edition.
Dinarello, C. A. (2004). Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J Endotoxin Res 10, 201-222. doi:10.1179/096805104225006129
Etna, M. P., Giacomini, E., Rizzo, F. et al. (2020). Optimization of the monocyte activation test for evaluating pyrogenicity of tick-borne encephalitis virus vaccine. ALTEX 37, 532-544. doi:10.14573/altex.2002252
Fomsgaard, A., Dinesen, B. and Baek, L. (1987). Anti-lipopolysaccharide antibodies measured by enzyme-immunoassay in Danish blood donors. Acta Pathol Microbiol Immunol Scand C 95, 9-13. doi:10.1111/j.1699-0463.1987.tb00002.x
Forte, T. M., Bell-Quint, J. J. and Cheng, F. (1981). Lipoproteins of fetal and newborn calves and adult steer: A study of developmental changes. Lipids 16, 240-245. doi:10.1007/bf02535023
Haylett, A. K. and Moore, J. V. (2002). Comparative analysis of foetal calf and human low density lipoprotein: Relevance for pharmacodynamics of photosensitizers. J Photochem Photobiol B 66, 171-178. doi:10.1016/s1011-1344(02)00241-5
Hoffmann, S., Peterbauer, A., Schindler, S. et al. (2005). International validation of novel pyrogen tests based on human monocytoid cells. J Immunol Methods 298, 161-173. doi:10.1016/j.jim.2005.01.010
Jaeger, M., Stappers, M. H., Joosten, L. A. et al. (2015). Genetic variation in pattern recognition receptors: Functional consequences and susceptibility to infectious disease. Future Microbiol 10, 989-1008. doi:10.2217/fmb.15.37
Kikkert, R., de Groot, E. R. and Aarden, L. A. (2008). Cytokine induction by pyrogens: Comparison of whole blood, mononuclear cells, and TLR-transfectants. J Immunol Methods 336, 45-55. doi:10.1016/j.jim.2008.03.010
Koryakina, A., Frey, E. and Bruegger, P. (2014). Cryopreservation of human monocytes for pharmacopeial monocyte activation test. J Immunol Methods 405, 181-191. doi:10.1016/j.jim.2014.01.005
Meszaros, K., Aberle, S., White, M. et al. (1995). Immunoreactivity and bioactivity of lipopolysaccharide-binding protein in normal and heat-inactivated sera. Infect Immun 63, 363-365. doi:10.1128/IAI.63.1.363-365.1995
Nys, M., Laub, R., Damas, P. et al. (1996). Screening and characterization of specific anti-lipopolysaccharide antibodies in Belgian blood donors by enzyme-linked immunosorbent assays. Eur J Clin Invest 26, 1134-1142. doi:10.1046/j.1365-2362.1996.500591.x
Park, B. S. and Lee, J. O. (2013). Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45, e66. doi:10.1038/emm.2013.97
Perdomo-Morales, R., Pardo-Ruiz, Z., Spreitzer, I. et al. (2011). Monocyte activation test (MAT) reliably detects pyrogens in parenteral formulations of human serum albumin. ALTEX 28, 227-235. doi:10.14573/altex.2011.3.227
Poole, S., Mistry, Y., Ball, C. et al. (2003). A rapid ‘one-plate’ in vitro test for pyrogens. J Immunol Methods 274, 209-220. doi:10.1016/s0022-1759(02)00519-7
Raetz, C. R. and Whitfield, C. (2002). Lipopolysaccharide endotoxins. Annu Rev Biochem 71, 635-700. doi:10.1146/annurev.biochem.71.110601.135414
Rahman, H., Qasim, M., Schultze, F. C. et al. (2011). Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome. Proteome Sci 9, 71. doi:10.1186/1477-5956-9-71
Rossi, O., Citiulo, F. and Mancini, F. (2020). Outer membrane vesicles: Moving within the intricate labyrinth of assays that can predict risks of reactogenicity in humans. Hum Vaccin Immunother 17, 601-613. doi:10.1080/21645515.2020.1780092
Schindler, S., Bristow, A., Cartmell, T. et al. (2003). Comparison of the reactivity of human and rabbit blood towards pyrogenic stimuli. ALTEX 20, 59-63. https://www.altex.org/index.php/altex/article/view/1045
Solati, S., Aarden, L., Zeerleder, S. et al. (2015). An improved monocyte activation test using cryopreserved pooled human mononuclear cells. Innate Immun 21, 677-684. doi:10.1177/1753425915583365
Soltis, R. D., Hasz, D., Morris, M. J. et al. (1979). The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology 36, 37-45.
van der Valk, J., Bieback, K., Buta, C. et al. (2018). Fetal bovine serum (FBS): Past – Present – Future. ALTEX 35, 99-118. doi:10.14573/altex.1705101
Vipond, C., Sutherland, J., Nordgren, K. et al. (2019). Development and validation of a monocyte activation test for the control/safety testing of an OMV-based meningococcal B vaccine. Vaccine 37, 3747-3753. doi:10.1016/j.vaccine.2018.06.038
Wendel, M., Paul, R. and Heller, A. R. (2007). Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med 33, 25-35. doi:10.1007/s00134-006-0433-x