Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood monocyte activation test
Main Article Content
Abstract
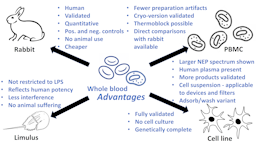
The whole blood pyrogen test invented 25 years ago, and its variant based on cryo-preserved blood one year later, brought momentum into the field of pyrogen testing, which, despite the broad application of the Limulus amebocyte lysate (LAL) assay, aka bacterial endotoxin test (BET), consumed several hundred thousand rabbits per year world-wide. The resulting international validation and lengthy acceptance and implementation process of what are called now monocyte activation tests (MATs) finally is impacting on animal numbers – at least in Europe – reducing them by more than 70% and counting. The author sees no reason for continuing any regulatory rabbit testing for pyrogens except the lack of acceptance of MATs in some regions of the world. The availability of MATs has opened also the discussion about the shortcomings of LAL/BET, namely its restriction to Gram-negative pyrogens, non-reflection of the potency of these in humans, interference and masking by many products, and animal welfare concerns for horseshoe crabs. The obvious advantages of MATs in all these respects should lead to a shift from LAL/BET to MATs. We are starting to see this for vaccines and medical devices, but other areas like safety testing of blood transfusions, cell therapies and nanomaterials, and the assessment of air-borne pyrogens still need to grasp the opportunity provided by MATs. While the different MATs can jointly serve these needs, the whole blood MAT has some advantages as discussed here.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Agzie, M., Niguse, S., Tsegay, E. et al. (2019). Bacterial contaminants of stored blood and blood components ready for transfusion at blood banks in Mekelle, Northern Ethiopia. BMC Res Notes 12, 169. doi:10.1186/s13104-019-4217-0
Alcaina, P. S. (2020). Platelet transfusion: An update on challenges and outcomes. J Blood Med 11, 19-26. doi:10.2147/jbm.s234374
Banerjee, S. and Mohanan, P. V. (2011). Inflammatory response to pyrogens determined by a novel ELISA method using human whole blood. J Immunol Methods 369, 146-153. doi:10.1016/j.jim.2011.05.004
Boneberg, E. and Hartung, T. (2003). Febrile temperatures attenuate IL-1 release by inhibiting proteolytic processing of the proform and influence Th1/Th2 balance favoring Th2 cytokines. J Immunol 171, 664-668. doi:10.4049/jimmunol.171.2.664
Borton, L. and Coleman, K. P. (2018). Material-mediated pyrogens in medical devices: Applicability of the in vitro monocyte activation test. ALTEX 35, 453-463. doi:10.14573/altex.1709221
Bottini, A. A., Amcoff, P. and Hartung, T. (2007). Food for thought … on globalization of alternative methods. ALTEX 24, 255-261. doi:10.14573/altex.2007.4.255
Bottini, A. A. and Hartung, T. (2009). Food for thought … on economics of animal testing. ALTEX 26, 3-16. doi:10.14573/altex.2009.1.3
Brecher, M. E. and Hay, S. N. (2005). Bacterial contamination of blood components. Clin Microbiol Rev 18, 195-204. doi:10.1128/cmr.18.1.195-204.2005
Brown, J., Clippinger, A. J., Goode, J. et al. (2021). Using the monocyte activation test as a stand-alone release test for medical devices: A workshop report. ALTEX 38, 151-156. doi:10.14573/altex.2012021
Bunk, S., Sigel, S., Metzdorf, D. et al. (2010). Internalization and coreceptor expression are critical for TLR2-mediated recognition of lipoteichoic acid in human peripheral blood. J Immunol 185, 3708-3717. doi:10.4049/jimmunol.0901660
Busquet, F., Kleensang, A., Rovida, C. et al. (2020). New European Union statistics on laboratory animal use – What really counts! ALTEX 37, 167-186. doi:10.14573/altex.2003241
Carlin, G. and Viitanen, E. (2005). In vitro pyrogenicity of the diphtheria, tetanus and acellular pertussis components of a trivalent vaccine. Vaccine 23, 3709-3715. doi:10.1016/j.vaccine.2005.02.010
Daneshian, M., Guenther, A., Wendel, A. et al. (2006). In vitro pyrogen test for toxic or immunomodulatory drugs. J Immunol Methods 313, 169-175. doi:10.1016/j.jim.2006.04.009
Daneshian, M., Wendel, A., Hartung, T. et al. (2008). High sensitivity pyrogen testing in water and dialysis solutions. J Immunol Methods 336, 64-70. doi:10.1016/j.jim.2008.03.013
Daneshian, M., von Aulock, S. and Hartung, T. (2009). Assessment of pyrogenic contaminations with the validated human whole blood assay. Nat Protoc 12, 1709-1721. doi:10.1038/nprot.2009.159
da Silva, C. C., Presgrave, O. A. F., Hartung, T. et al. (2015). Applicability of the monocyte activation test (MAT) for hyperimmune sera in the routine of the quality control laboratory: Comparison with the rabbit pyrogen test (RPT). Toxicol In Vitro 32, 70-75. doi:10.1016/j.tiv.2015.12.004
Dehus, O., Hartung, T. and Hermann, C. (2006). Endotoxin evaluation of eleven lipopolysaccharides by whole blood assay does not always correlate with Limulus amebocyte lysate assay. J Endotoxin Res 12, 71-80. doi:10.1179/096805106X102156
de Mattos, K. A., Navega, E. C. A., Silva, V. F. et al. (2018). Applicability of the monocyte activation test (MAT) in the quality control of the 17DD yellow fever vaccine. Altern Lab Anim 46, 23-37. doi:10.1177/026119291804600107
Ding, J. L. and Ho, B. (2010). Endotoxin detection – From Limulus amebocyte lysate to recombinant factor C. Subcell Biochem 53, 187-208. doi:10.1007/978-90-481-9078-2_9
Dobrovolskaia, M. A., Neun, B. W., Clogston, J. D. et al. (2010). Ambiguities in applying traditional Limulus amebocyte lysate tests to quantify endotoxin in nanoparticle formulations. Nanomedicine 5, 555-562. doi:10.2217/nnm.10.29
Etna, M. P., Giacomini, E., Rizzo, F. et al. (2020). Optimization of the monocyte activation test for evaluating pyrogenicity of tick-borne encephalitis virus vaccine. ALTEX 37, 532-544. doi:10.14573/altex.2002252
Fan, X., Stelter, F., Menzel, R. et al. (1999). Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect Immun 67, 2964-2968. doi:10.1128/IAI.67.6.2964-2968.1999
Fennrich, S., Fischer, M., Hartung, T. et al. (1999a). Detection of endotoxins and other pyrogens using human whole blood. Dev Biol Stand 101, 131-139.
Fennrich, S., Wendel, A. and Hartung, T. (1999b). New applications of the human whole blood pyrogen assay (PyroCheck). ALTEX 16, 146-149. https://www.altex.org/index.php/altex/article/view/1453
Fennrich, S., Hennig, U., Toliashvili, L. et al. (2016). More than 70 years of pyrogen detection: Current state and future perspectives. Altern Lab Anim 44, 239-253. doi:10.1177/026119291604400305
Frattini, A., Fabbri, M., Valli, R. et al. (2015). High variability of genomic instability and gene expression profiling in different HeLa clones. Sci Rep 5, 15377. doi:10.1038/srep15377
Gerke, C., Colucci, A. M., Giannelli, C. et al. (2015). Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One 10, e0134478. doi:10.1371/journal.pone.0134478
Gimenes, I., Caldeira, C., Presgrave, O. A. F. et al. (2015). Assessment of pyrogenic response of lipoteichoic acid by the monocyte activation test and the rabbit pyrogen test. Regulat Toxicol Pharmacol 73, 356-360. doi:10.1016/j.yrtph.2015.07.025
Grallert, H., Leopoldseder, S., Schuett, M. et al. (2011). EndoLISA®: A novel and reliable method for endotoxin detection. Nature Meth 8, iii-iv. doi:10.1038/nmeth.f.350
Hartung, T. and Wendel, A. (1995). Die Erfassung von Pyrogenen in einem humanen Vollblutmodell (Detection of pyrogens using human whole blood). ALTEX 12, 70-75. https://www.altex.org/index.php/altex/article/view/1671
Hartung, T. and Wendel, A. (1996). Detection of pyrogens using human whole blood. In Vitro Toxicol 9, 353-359.
Hartung, T., Aaberge, I., Berthold, S. et al. (2001). ECVAM workshop on novel pyrogen tests based on the human fever reaction. Altern Lab Anim 29, 99-123. doi:10.1177/026119290102900203
Hartung, T. (2010a). Food for thought … on alternative methods for nanoparticle safety testing. ALTEX 27, 87-95. doi:10.14573/altex.2010.2.87
Hartung, T. (2010b). Comparative analysis of the revised Directive 2010/63/EU for the protection of laboratory animals with its predecessor 86/609/EEC – A t4 report. ALTEX 27, 285-303. doi:10.14573/altex.2010.4.285
Hartung, T. and Sabbioni, E. (2011). Alternative in vitro assays in nanomaterial toxicology. WIREs Nanomed Nanobiotechnol 3, 545-573. doi:10.1002/wnan.153
Hartung, T. (2013). Look back in anger – What clinical studies tell us about preclinical work. ALTEX 30, 275-291. doi:10.14573/altex.2013.3.275
Hartung, T. (2015). The human whole blood pyrogen test – Lessons learned in twenty years. ALTEX 32, 79-100. doi:10.14573/altex.1503241
Hasiwa, M., Kullmann, K., von Aulock, S. et al. (2007). An in vitro pyrogen safety test for immune-stimulating components on surfaces. Biomat 28, 1367-1375. doi:10.1016/j.biomaterials.2006.11.016
Hasiwa, N., Daneshian, M., Bruegger, P. et al. (2013). Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX 30, 169-208. doi:10.14573/altex.2013.2.169
He, Q., Gao, H., Xu, L. et al. (2018). Analysis of IL-6 and IL-1β release in cryopreserved pooled human whole blood stimulated with endotoxin. Innate Immun 24, 316-322. doi:10.1177/1753425918777596
Hermann, C., Spreitzer, I., Schröder, N. W. J. et al. (2002). Cytokine induction by purified lipoteichoic acids from various bacterial species – Role of LBP, sCD14, CD14 and failure to induce interleukin-12 and subsequent interferon-gamma release. Eur J Immunol 32, 541-551. doi:10.1002/1521-4141(200202)32:2<541::AID-IMMU541>3.0.CO;2-P
Hoffmann, S., Peterbauer, A., Schindler, S. et al. (2005). International validation of novel pyrogen tests based on the human fever reaction. J Immunol Methods 298, 161-173. doi:10.1016/j.jim.2005.01.010
Huang, L. Y., DuMontelle, J. L., Zolodz, M. et al. (2009). Use of toll-like receptor assays to detect and identify microbial contaminants in biological products. J Clin Microbiol 47, 3427-3434. doi:10.1128/jcm.00373-09
Ip, W. K., Takahashi, K., Moore, K. J. et al. (2008). Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med 205, 169-181. doi:10.1084/jem.20071164
ISO 10993-1 (2009). International Standard. Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process. https://www.iso.org/standard/44908.html
Jimenez-Dalmaroni, M. J., Xiao, N., Corper, A.L. et al. (2009). Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS One 4, e7411. doi:10.1371/journal.pone.0007411
Jin, Y., Jia, J., Li, C. et al. (2018). LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEG2000 nanoformulation: What if traditional methods are not applicable? Asian J Pharmaceut Sci 13, 289-296. doi:10.1016/j.ajps.2017.11.003
Kerecman Myers, D., Goldberg, A. M., Poth, A. et al. (2017). From in vivo to in vitro: The medical device testing paradigm shift. ALTEX 34, 479-500. doi:10.14573/altex.1608081
Kindinger, I., Fennrich, S., Zucker, B. et al. (2002). Determination of air-borne pyrogens by the in vitro pyrogen test (IPT) based on human whole blood cytokine response. VDI Bericht 1656, 499-507.
Kindinger, I., Daneshian, M., Baur, H. et al. (2005). A new method to measure air-borne pyrogens based on human whole blood cytokine response. J Immunol Methods 298, 143-153. doi:10.1016/j.jim.2005.01.006
Kleensang, A., Vantangoli, M. M., Odwin-DaCosta, S. et al. (2015). Genetic variability in a frozen batch of MCF-7 cells invisible in routine authentication affecting cell function. Sci Rep 6, 28994-28994. doi:10.1038/srep28994
Koryakina, A., Frey, E. and Bruegger, P. (2014). Cryopreservation of human monocytes for pharmacopeial monocyte activation test. J Immunol Methods 405, 181-191. doi:10.1016/j.jim.2014.01.005
Kretzschmar, E., Muckenfuss, H., and Pfleiderer, M. (2018). Official batch control of influenza vaccines: Is it still useful? Vaccine 36, 2364-2370. doi:10.1016/j.vaccine.2018.02.078
Li, Y. and Boraschi, D. (2016). Endotoxin contamination: A key element in the interpretation of nanosafety studies. Nanomed 11, 269-287 doi:10.2217/nnm.15.196
Li, Y., Fujita, M. and Boraschi, D. (2017a). Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results. Front Immunol 8, 472. doi:10.3389/fimmu.2017.00472
Li, Y., Shi, Z., Radauer-Preiml, I. et al. (2017b). Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation, Nanotoxicol 11, 1157-1175, doi:10.1080/17435390.2017.1401142
Lynch, N. J., Roscher, S., Hartung, T. et al. (2004). L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol 172, 1198-1202. doi:10.4049/jimmunol.172.2.1198
Mazzotti, F., Beuttler, J., Zeller, R. et al. (2007). In vitro pyrogen test – A new test method for solid medical devices. J Biomed Mater Res 80, 276-282. doi:10.1002/jbm.a.30922
Meigs, L., Smirnova, L., Rovida, C. et al. (2018). Animal testing and its alternatives – The most important omics is economics. ALTEX 35, 275-305. doi:10.14573/altex.1807041
Molenaar-de Backer, M., Gitz, E., Dieker, M. et al. (2021). Performance of monocyte activation test supplemented with human serum compared to fetal bovine serum. ALTEX, online ahead of print. doi:10.14573/altex.2008261
Morath, S., Geyer, A., Spreitzer, I. et al. (2002). Structural decomposition and heterogeneity of commercial lipoteichoic acid preparation. Infect Immun 70, 938-944. doi:10.1128/iai.70.2.938-944.2002
Negherbon, J. P., Romero, K., Williams, D. L. et al. (2017). Whole blood cytokine response to local traffic-related particulate matter in Peruvian children with and without asthma. Front Pharmacol 8, 157. doi:10.3389/fphar.2017.00157
Neun, B. W. and Dobrovolskaia, M. A. (2017). Characterization of nanoparticles intended for drug delivery. Methods Mol Biol 1682, 23-33. doi:10.1007/978-1-4939-7352-1_3
Ørving, R. B., Carpenter, B., Roth, S. et al. (2020). Bacterial endotoxin testing – Fast endotoxin masking kinetics in the presence of lauryldimethylamine oxide. Microorganisms 8, 1728. doi:10.3390/microorganisms8111728
Osler, W. (1896). The study of the fevers of the South. JAMA 26, 999-1004. doi:10.1001/jama.1896.02430730001001
Reich, J., Lang, P., Grallert, H. et al. (2016). Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 44, 417-422. doi:10.1016/j.biologicals.2016.04.012
Reich, J., Tamura, H., Nagaoka, I. et al. (2018). Investigation of the kinetics and mechanism of low endotoxin recovery in a matrix for biopharmaceutical drug products. Biologicals 53, 1-9. doi:10.1016/j.biologicals.2018.04.001
Reich, J., Weyer, F. A., Tamura, H. et al. (2019). Low endotoxin recovery – Masking of naturally occurring endotoxin. Int J Mol Sci 20, 838. doi:10.3390/ijms20040838
Rockel, C. and Hartung, T. (2012). Systematic review of membrane components of Gram-positive bacteria responsible as pyrogens for inducing human monocyte / macrophage cytokine release. Front Pharmacol 3, 56. doi:10.3389/fphar.2012.00056
Rossi, O., Citiulo, F. and Mancini, F. (2020). Outer membrane vesicles: Moving within the intricate labyrinth of assays that can predict risks of reactogenicity in humans. Hum Vaccin Immunother. doi:10.1080/21645515.2020.1780092
Schindler, S., Bristow, A., Cartmell, T. et al. (2003). Comparison of the reactivity of human and rabbit blood towards pyrogenic stimuli. ALTEX 20, 59-63. https://www.altex.org/index.php/altex/article/view/1045
Schindler, S., Spreitzer, I., Loschner, B. et al. (2006a). International validation of pyrogen tests based on cryopreserved human primary blood cells. J Immunol Methods 316, 42-51. doi:10.1016/j.jim.2006.07.023
Schindler, S., Rosenberg, U., Schlote, D. et al. (2006b). Pyrogen testing of lipidic parenterals with a novel in vitro test. J Pharmeur Sci Notes 2006, 1-7.
Schindler, S., von Aulock, S., Daneshian, M. et al. (2009). Development, validation and applications of the monocyte activation test for pyrogens based on human whole blood. ALTEX 26, 93-305. doi:10.14573/altex.2009.4.265
Schröder, N. W. J., Morath, S., Alexander, C. et al. (2003). Lipoteichoic acid (LTA) of S. pneumoniae and S. aureus activates immune cells via toll-like receptor (TLR)-2, LPS binding protein (LBP) and CD14 while TLR-4 and MD-2 are not involved. J Biol Chem 278, 15587-15594. doi:10.1074/jbc.M212829200
Schwarz, H., Gornicec, J., Neuper, T. et al. (2017). Biological activity of masked endotoxin. Sci Rep 7, 44750. doi:10.1038/srep44750
Sigel, S., Bunk, S., Meergans, T. et al. (2012). Apolipoprotein B100 is a suppressor of Staphylococcus aureus-induced innate immune responses in humans and mice. Eur J Immunol 42, 2983-2989. doi:10.1002/eji.201242564
Silva, V. F., da Silva Guedes D. Jr., da Silveira, I. A., et al. (2018). A comparison of pyrogen detection tests in the quality control of meningococcal conjugate vaccines: The applicability of the Monocyte Activation Test. Altern Lab Anim 46, 255-272. doi:10.1177/026119291804600506
Solati, S., Aarden, L., Zeerleder, S. et al. (2015). An improved monocyte activation test using cryopreserved pooled human mononuclear cells. Innate Immun 21, 677-684. doi:10.1177/1753425915583365
Spoladore, J., Gimenes Lopes, I., Bachinski, R. F. et al. (submitted). Standardized pyrogen testing of health products with bacterial endotoxin Test (BET) as a substitute for rabbit pyrogen testing (RPT): A scoping review.
Spreitzer, I., (2019). Evolution and characteristics of the monocyte activation test (MAT). In K. L. Williams (ed.), Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems (523-535). Cham, Switzerland: Springer Nature Switzerland AG. doi:10.1007/978-3-030-17148-3_14
Stang, K., Fennrich, S., Krajewski, S. et al. (2014). Highly sensitive pyrogen detection on medical devices by the monocyte activation test. J Mater Sci Mater Med 25, 1065-1075. doi:10.1007/s10856-013-5136-6
Stoddard, M. B., Pinto, V., Keiser, P. B. et al. (2010). Evaluation of a whole-blood cytokine release assay for use in measuring endotoxin activity of group B Neisseria meningitidis vaccines made from lipid A acylation mutants. Clin Vaccine Immunol 17, 98-107. doi:10.1128/CVI.00342-09
Stoppelkamp, S., Würschum, N., Stang, K. et al. (2017). Speeding up pyrogenicity testing: Identification of suitable cell components and readout parameters for an accelerated monocyte activation test (MAT). Drug Test Anal 9, 260-273. doi:10.1002/dta.1973
Stormer, M., Wood, M. and Gathof, B. (2019). Microbial safety of cellular therapeutics – Lessons from over ten years’ experience in microbial safety of platelet concentrates. ISBT Sci Ser 14, 37-44. doi:10.1111/voxs.12452
Studholme, L., Sutherland, J., Desai, T. et al. (2019). Evaluation of the monocyte activation test for the safety testing of meningococcal B vaccine Bexsero: A collaborative study. Vaccine 37, 3761-3769. doi:10.1016/j.vaccine.2018.05.073
USP – United States Pharmacopeia (2017). <151> Pyrogen Test. In U.S. Pharmacopeia 40, United States Pharmacopeial Convention, Inc.: Rockville, MD, USA.
Utescher, C. L. d. A., Buosi, K. L., Botosso, V. F. et al. (2018). Monocyte activation test (MAT) as a possibility of replacement for the rabbit pyrogen test in hyperimmune sera. Braz J Pharm Sci 54, e17530. doi:10.1590/s2175-97902018000217530
Valentini, S., Santoro, G., Baffetta, F. et al. (2018). Monocyte-activation test to reliably measure the pyrogenic content of a vaccine: An in vitro pyrogen test to overcome in vivo limitations. Vaccine 37, 3754-3760. doi:10.1016/j.vaccine.2018.10.082
Vipond, C., Findlay, L., Feavers, I. et al. (2016). Limitations of the rabbit pyrogen test for assessing meningococcal OMV based vaccines. ALTEX 33, 47-53. doi:10.14573/altex.1509291
Vipond, C., Sutherland, J., Nordgren, K. et al. (2019). Development and validation of a monocyte activation test for the control/safety testing of an OMV-based meningococcal B vaccine. Vaccine 37, 3747-3753. doi:10.1016/j.vaccine.2018.06.038
von Aulock, S., Deininger, S., Draing, C. et al. (2006). Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res 26, 887-892. doi:10.1089/jir.2006.26.887
Westphal, O., Lüderitz, O. and Bister, F. (1952). Über die Extraktion von Bakterien mit Phenol/Wasser. Zeitschrift für Naturforschung B 7, 148-155. doi:10.1515/znb-1952-0303
Zervos, C., Zimmerman, T. P., Willis, T. et al. (2019). Immunoglobulin G from single plasma donor in immune globulin intravenous causes false positive pyrogen test. Biologicals 59, 12-19. doi:10.1016/j.biologicals.2019.04.001