Animal metrics: Tracking contributions of new approach methods to reduced animal use
Main Article Content
Abstract
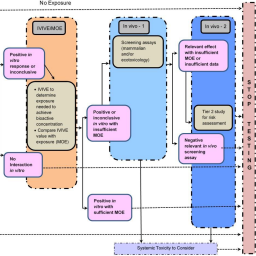
Many companies and global regulatory programs have expressed the intent to move away from in vivo animal testing to new approach methods (NAMs) as part of product safety assessments. NAMs, which include non-animal approaches for testing and assessment – from computer-based modeling to in chemico or in vitro models – allow faster data generation with potentially greater relevance to humans while avoiding animal use. To monitor progress implementing NAMs, each organization first must define what is in scope, starting with the definition of “animal” (e.g., mammals, vertebrates) and applicable studies (e.g., animals used for “in-house” experiments, at contract research organizations, as part of environmental monitoring). Next, organizations must establish baseline animal use, including defined rules for inclusion/ exclusion of animals that ensure consistency in future assessments. Lastly, organizations must establish metrics for animal savings based on the utility of NAM data. This paper presents one approach to establish “animal use” metrics in a toxicology program at The Dow Chemical Company. The premise of our program is that most NAM information has value for animal savings, but the value depends on how data are used (e.g., research and development, screening, or regulatory requirements) and the level of certainty for internal decision-making. This manuscript provides metrics on the impact of NAMs, allowing a quantitative assessment of animal use numbers over time, accountability for resources spent on NAM development, and identification of areas where NAM development is still needed. This approach can be refined for use at other organizations.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Archibald, K., Drake T. and Coleman, R. (2015). Barriers to the uptake of human-based test methods, and how to overcome them. Altern Lab Anim 43, 301-308. doi:10.1177/026119291504300504
Ball, N., Cronin M. T. D., Shen J. et al. (2016). Toward good read-across practice (GRAP) guidance. ALTEX 33, 149-166. doi:10.14573/altex.1601251
Baltazar, M. T., Cable, S., Carmichael, P. L. et al. (2020). A next-generation risk assessment case study for coumarin in cosmetic products. Toxicol Sci 176, 236-252. doi:10.1093/toxsci/kfaa048
Bartlett, D. H. and Silk, S. B. (2016). Office of Laboratory Animal Welfare Comments. Zebrafish 13, 563-564. doi:10.1089/zeb.2016.1344
Bhhatarai, B., Wilson, D. M., Bartels, M. J. et al. (2015). Acute toxicity prediction in multiple species by leveraging mechanistic ToxCast mitochondrial inhibition data and simulation of oral bioavailability. Toxicol Sci 147, 386-396. doi:10.1093/toxsci/kfv135
Chary, A., Hennen, J., Klein, S. G. et al. (2018). Respiratory sensitization: Toxicological point of view on the available assays. Arch Toxicol 92, 803-822. doi:10.1007/s00204-017-2088-5
Chary, A., Serchi, T., Moschini, E. et al. (2019). An in vitro coculture system for the detection of sensitization following aerosol exposure. ALTEX 36, 403-418. doi:10.14573/altex.1901241
Clippinger, A. J., Hill, E., Curren, R. et al. (2016). Bridging the gap between regulatory acceptance and industry use of non-animal methods. ALTEX 33, 453-458. doi:10.14573/altex.1601311
Clippinger, A. J., Raabe, H. A., Allen, D. G. et al. (2021). Human-relevant approaches to assess eye corrosion/irritation potential of agrochemical formulations. Cutan Ocul Toxicol 40, 145-167. doi:10.1080/15569527.2021.1910291
Corvaro, M., Gehen, S., Andrews, K. et al. (2016). GHS additivity formula: A true replacement method for acute systemic toxicity testing of agrochemical formulations. Regul Toxicol Pharmacol 82, 99-110. doi:10.1016/j.yrtph.2016.10.007
Dalvie, D., Obach, R. S., Kang, P. et al. (2009). Assessment of three human in vitro systems in the generation of major human excretory and circulating metabolites. Chem Res Toxicol 22, 357-368. doi:10.1021/tx8004357
EC – European Commission (2020). Report from the Commission to the European Parliament and the Council. 2019 report on the statistics on the use of animals for scientific purposes in the Member States of the European Union in 2015-2017. European Commission, Brussels 5.2.2020. Report: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52020DC0016&from=EN; Website: https://ec.europa.eu/environment/chemicals/lab_animals/reports_en.htm (accessed 17.07.2021)
EFSA – European Food Safety Authority (2014). Modern methodologies and tools for human hazard assessment of chemicals. Scientific report of EFSA. EFSA J 12, 3638. doi:10.2903/j.efsa.2014.3638
EU – European Union (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. European Union (EU). https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02009R1223-20190813&from=EN#tocId2 (accessed 17.07.2021)
EU (2010). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJ L 276, 33-79. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32010L0063&from=EN (accessed 17.07.2021)
EURL ECVAM – EU Reference Laboratory for Alternatives to Animal Testing (2020). EURL ECVAM Recommendation on Non-Animal-Derived Antibodies. doi:10.2760/80554
Groff, K., Allen, D., Casey, W. et al. (2020). Increasing the use of animal-free recombinant antibodies. ALTEX 37, 309-311. doi:10.14573/altex.2001071
ICCVAM – Interagency Coordinating Committee on the Validation of Alternative Methods (2018). A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States. https://ntp.niehs.nih.gov/whatwestudy/niceatm/natl-strategy/index.html (accessed 17.07.2021)
ICCVAM (2021). ICCVAM Metrics Workgroup. Measuring U.S. Federal Agency Progress Toward Implementation of Alternative Methods in Toxicity Testing. https://ntp.niehs.nih.gov/iccvam/docs/about_docs/iccvam-measuringprogress-feb2021-fd-508.pdf (accessed 17.07.2021)
Kleinstreuer, N. C., Hoffmann, S., Alépée, N. et al. (2018). Non-animal methods to predict skin sensitization (II): An assessment of defined approaches*. Crit Rev Toxicol 48, 359-374. doi:10.1080/10408444.2018.1429386
Krebs, A., van Vugt-Lussenburg, B. M. A., Waldmann, T. et al. (2020). The EU-ToxRisk method documentation, data processing and chemical testing pipeline for the regulatory use of new approach methods. Arch Toxicol 94, 2435-2461. doi:10.1007/s00204-020-02802-6
Krieger, S., Auernhammer, T., Hotchkiss, J. et al. (submitted). Mechanistic profiling of inhalation sensory irritants with a computational model for transient receptor potential vanilloid subfamily type 1 (TRPV1).
Ladics, G. S., Smith, C., Heaps, K. et al. (1995). Possible incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats on standard toxicology study. Toxicology 96, 225-238. doi:10.1016/0300-483x(94)02967-y
Leist, M., Efremova, L. and Karreman, C. (2010). Food for thought considerations and guidelines for basic test method descriptions in toxicology. ALTEX 27, 309-317. doi:10.14573/altex.2010.4.309
Luechtefeld, T., Maertens, A., Russo, D. P. et al. (2016). Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data. ALTEX 33, 123-134. doi:10.14573/altex.1510053
Luechtefeld, T., Marsh, D., Rowlands, C. et al. (2018). Machine learning of toxicological big data enables read-across structure activity relationships (RASAR) outperforming animal test reproducibility. Toxicol Sci 165, 198-212. doi:10.1093/toxsci/kfy152
Lillicrap, A., Belanger, S., Burden, N. et al. (2016). Alternative approaches to vertebrate ecotoxicity tests in the 21st century: A review of developments over the last 2 decades and current status. Environ Toxicol Chem 35, 2637-2646. doi:10.1002/etc.3603
Luccio-Camelo, D. C. and Prins, G. S. (2011). Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Biol 127, 74-82. doi:10.1016/j.jsbmb.2011.04.004
Manolis, E., Vamvakas, S. and Isaac, M. (2011). New pathway for qualification of novel methodologies in the European medicines agency. Proteomics Clin Appl 5, 248-255. doi:10.1002/prca.201000130
Mansouri, K., Kleinstreuer, N., Abdelaziz, A. M. et al. (2020). COMPARA: Collaborative modeling project for androgen receptor activity. Environ Health Perspect 128, 27002. doi:10.1289/EHP5580
Meigs, L., Smirnova, L., Rovida, C. et al. (2018). Animal testing and its alternatives – The most important omics is economics. ALTEX 35, 275-305. doi:10.14573/altex.1807041
NIH – National Institutes of Health (2021). Guidelines for Preparing USDA Annual Reports and Assigning USDA Reporting Columns. https://oacu.oir.nih.gov/system/files/media/file/2021-02/a1-usda.pdf (accessed 23.11.2021)
NRC (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC, USA: The National Academies Press. doi:10.17226/11970
OECD – Organisation for Economic Cooperation and Development (2009). Test No. 441: Hershberger Bioassay in Rats: A Short-Term Screening Assay for (Anti)Androgenic Properties. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264076334-en
OECD (2010). Test No. 429: Skin Sensitization: Local Lymph Node Assay. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264071100-en
OECD (2015). Adverse Outcome Pathways, Molecular Screening and Toxicogenomics. OECD Testing of Chemicals. OECD, Paris, France. https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm (accessed 17.07.2021)
OECD (2016). Integrated Approaches to Testing and Assessment (IATA). OECD Assessment of Chemicals. OECD, Paris, France. http://www.oecd.org/chemicalsafety/risk-assessment/iata-integrated-approaches-to-testing-and-assessment.htm (accessed 17.07.2021)
OECD (2017). Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitization. OECD Series on Testing and Assessment, No. 256. OECD Publishing, Paris. doi:10.1787/9789264279285-en
OECD (2018a). Test 443: Extended One-Generation Reproductive Toxicity Study. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264185371-en
OECD (2018b). Guidance Document on Good In Vitro Methods Practices (GIVIMP). OECD Series on Testing and Assessment, No. 286. OECD Publishing, Paris. doi:10.1787/20777876
OECD (2021). Guideline No. 497: Defined Approaches on Skin Sensitization. OECD Guideline for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/b92879a4-en
Pham, L. L., Watford, S. M., Pradeep, P. et al. (2020). Variability in in vivo studies: Defining the upper limit of performance for predictions of systemic effect levels. Comput Toxicol 15, 100126. doi:10.1016/j.comtox.2020.100126
Rooney, J. P., Choksi, N. Y., Ceger, P. et al. (2021). Analysis of variability in the rabbit skin irritation assay. Regul Toxicol Pharmacol 122, 104920. doi:10.1016/j.yrtph.2021.104920
Rovida, C. and Hartung, T. (2009). Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals – A report by the transatlantic think tank for toxicology (t4). ALTEX 26, 187-208. doi:10.14573/altex.2009.3.187
Schmidt, B. Z., Lehmann, M., Gutbier, S. et al. (2017). In vitro acute and developmental neurotoxicity screening: An overview of cellular platforms and high-throughput technical possibilities. Arch Toxicol 91, 1-33. doi:10.1007/s00204-016-1805-9
Terry, C., Rasoulpour, R. J., Saghir, S. et al. (2014). Application of a novel integrated toxicity testing strategy incorporating “3R” principles of animal research to evaluate the safety of a new agrochemical sulfoxaflor. Crit Rev Toxicol 44, 1-14. doi:10.3109/10408444.2014.910753
US EPA – US Environmental Protection Agency (2018). Strategic Plan to Promote the Development and Implementation of Alternative Test Methods Within the TSCA Program. June 22, 2018. https://www.epa.gov/sites/production/files/2018-06/documents/epa_alt_strat_plan_6-20-18_clean_final.pdf (accessed 17.07.2021)
US EPA (2019a). Administrator Wheeler Signs Memo to Reduce Animal Testing, Awards $4.25 Million to Advance Research on Alternative Methods to Animal Testing. September 10, 2019. https://www.epa.gov/newsreleases/administrator-wheeler-signs-memo-reduce-animal-testing-awards-425-million-advance (accessed 26.09.2021)
US EPA (2019b). Administrator Memo Prioritizing Efforts to Reduce Animal Testing, September 10, 2019. https://www.epa.gov/research/administrator-memo-prioritizing-efforts-reduce-animal-testing-september-10-2019 (accessed 17.07.2021)
US GAO – US Government Accountability Office (2019). Animal Use in Research: Federal Agencies Should Assess and Report on Their Efforts to Develop and Promote Alternatives. GAO-19-629. https://www.gao.gov/products/gao-19-629 (accessed 17.07.2021)
van der Laan, J. W., Chapin, R. E., Haenen, B. et al. (2012). Testing strategies for embryo-fetal toxicity of human pharmaceuticals. Animal models vs. in vitro approaches. Regul Toxicol Pharmacol 63, 115-123. doi:10.1016/j.yrtph.2012.03.009
van der Valk, J., Mellor, D., Brands, R. et al. (2004). The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro 18, 1-12. doi:10.1016/j.tiv.2003.08.009
van der Valk, J., Brunner, D., De Smet, K. et al. (2010). Optimization of chemically defined cell culture media – Replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 24, 1053-1063. doi:10.1016/j.tiv.2010.03.016
van der Valk, J., Bieback, K., Buta, C. et al. (2018). Fetal Bovine Serum (FBS): Past – Present – Future. ALTEX 35, 99-118. doi:10.14573/altex.1705101
Wilson, D., Wijeyesakere, S. J., Parks, A. K. et al. (2018). Profiling acute oral and inhalation toxicity data using a computational workflow to screen for facile chemical reactivity. Appl In Vitro Toxicol 4, 214-219. doi:10.1089/aivt.2017.0041
Wijeyesakere, S., Wilson, D., Settivari, R. et al. (2018). Development of a profiler for facile chemical reactivity using the open-source Konstanz information miner. Appl In Vitro Toxicol 4, 202-213. doi:10.1089/aivt.2017.0040
Wijeyesakere, S. J., Wilson, D., Auernhammer, T. et al. (2019). Hybrid machine-learning/SMARTS profiling model for mitochondrial inhibition. Appl In Vitro Toxicol 5, 196-204. doi:10.1089/aivt.2019.0010
Wijeyesakere, S. J., Wilson, D. M. and Marty, M. S. (2020). Prediction of cholinergic compounds by machine learning. Comput Toxicol 13, 100119. doi:10.1016/j.comtox.2020.100119
Zhang, F., Bartels, M., Clark, A. et al. (2018). Performance evaluation of the GastroPlus™ software tool for prediction of the toxicokinetic parameters of chemicals. SAR QSAR Environ Res 29, 875-893. doi:10.1080/1062936X.2018.1518928