Comparison of human recombinant protein coatings and fibroblast-ECM to Matrigel for induced pluripotent stem cell culture and renal podocyte differentiation
Main Article Content
Abstract
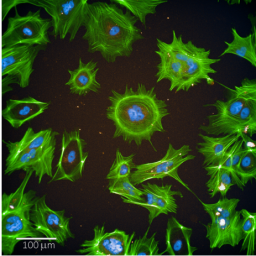
Human induced pluripotent stem cells (hiPSCs) offer great opportunities within the 3R framework. In the field of toxicology, they may contribute greatly to the reduction and eventually replacement of animal models. However, culturing hiPSCs as well as differentiation of hiPSCs into target cells that are used for toxicity testing depend on the presence of extracellular matrix (ECM) coating the growth surface. The most widely used ECM is MatrigelR, an animal product that is derived from mouse sarcoma. Drawbacks of Matrigel are widely recognized and include batch-to batch variations, use of animal rather than human material, and ethical concerns about its production. While alternative coatings exist, higher cost and limited characterizations may hinder their broader uptake by the scientific community. Here, we report an extensive comparison of three commercially available human ECM coatings, vitronectin, laminin-511, and laminin-521, to Matrigel in three different hiPSC lines in long-term culture (≥ 9 passages). Characterization included expression of pluripotent markers in a genome-wide transcriptomics study (TempO-Seq), capacity to differentiate into embryoid bodies, and karyotype stability assessed by analyzing copy number variations by shallow DNA sequencing. Furthermore, a low-cost, decellularized ECM produced by human neonatal dermal fibroblasts was tested. In addition, all alternative coatings were tested for hiPSC differentiation into renal podocyte-like cells in a genome-wide transcriptomics screen. Our results show that all tested coatings were highly comparable to animal-derived Matrigel for both hiPSC maintenance and differentiation into renal podocyte-like cells. Furthermore, decellularized fibroblast-ECM could be a novel, attractive low-cost coating material.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Albalushi, H., Kurek, M., Karlsson, L. et al. (2018). Laminin 521 stabilizes the pluripotency expression pattern of human embryonic stem cells initially derived on feeder cells. Stem Cells Int 2018, 7127042. doi:10.1155/2018/7127042
Björnson Granqvist, A., Ebefors, K., Saleem, M. A. et al. (2006). Podocyte proteoglycan synthesis is involved in the development of nephrotic syndrome. Am J Physiol Renal Physiol 291, F722-F730. doi:10.1152/ajprenal.00433.2005
Braam, S. R., Zeinstra, L., Litjens, S. et al. (2008). Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 26, 2257-2265. doi:10.1634/stemcells.2008-0291
Burgeson, R. E., Chiquet, M., Deutzmann, R. et al. (1994). A new nomenclature for the laminins. Matrix Biol 14, 209-211. doi:10.1016/0945-053x(94)90184-8
Burridge, P. W., Matsa, E., Shukla, P. et al. (2014). Chemically defined generation of human cardiomyocytes. Nat Methods 11, 855-860. doi:10.1038/nmeth.2999
Chandrasekaran, V., Carta, G., da Costa Pereira, D. et al. (2021). Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci Rep 11, 11575. doi:10.1038/s41598-021-89550-4
Chen, G., Gulbranson, D. R., Hou, Z. et al. (2011). Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8, 424-429. doi:10.1038/nmeth.1593
Chichagova, V., Sanchez-Vera, I., Armstrong, L. et al. (2016). Generation of human induced pluripotent stem cells using RNA-based Sendai virus system and pluripotency validation of the resulting cell population. Methods Mol Biol 1353, 285-307. doi:10.1007/7651_2015_205
Fergus, J., Quintanilla, R. and Lakshmipathy, U. (2016). Characterizing pluripotent stem cells using the TaqMan® hPSC scorecard™ panel. Methods Mol Biol 1307, 25-37. doi:10.1007/7651_2014_109
Frantz, C., Stewart, K. M. and Weaver, V. M. (2010). The extracellular matrix at a glance. J Cell Sci 123, 4195-4200. doi:10.1242/jcs.023820
Garitaonandia, I., Amir, H., Boscolo, F. S. et al. (2015). Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS One 10, e0118307. doi:10.1371/journal.pone.0118307
Hammad, S. M., Twal, W. O., Arif, E. et al. (2020). Transcriptomics reveal altered metabolic and signaling pathways in podocytes exposed to C16 ceramide-enriched lipoproteins. Genes (Basel) 11, 178. doi:10.3390/genes11020178
Hayman, E. G., Pierschbacher, M. D., Öhgren, Y. et al. (1983). Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci U S A 80, 4003-4007. doi:10.1073/pnas.80.13.4003
Hey, C. A. B., Saltõkova, K. B., Bisgaard, H. C. et al. (2018). Comparison of two different culture conditions for derivation of early hiPSC. Cell Biol Int 42, 1467-1473. doi:10.1002/cbin.10966
Holmes, R. (1967). Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol 32, 297-308. doi:10.1083/jcb.32.2.297
Hughes, C. S., Postovit, L. M. and Lajoie, G. A. (2010). Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886-1890. doi:10.1002/pmic.200900758
Hyslop, L., Stojkovic, M., Armstrong, L. et al. (2005). Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells 23, 1035-1043. doi:10.1634/stemcells.2005-0080
Hyvärinen, T., Hyysalo, A., Kapucu, F. E. et al. (2019). Functional characterization of human pluripotent stem cell-derived cortical networks differentiated on laminin-521 substrate: Comparison to rat cortical cultures. Sci Rep 9, 17125. doi:10.1038/s41598-019-53647-8
Imasawa, T., Obre, E., Bellance, N. et al. (2017). High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J 31, 294-307. doi:10.1096/fj.201600293r
Inoue, H., Nagata, N., Kurokawa, H. et al. (2014). IPS cells: A game changer for future medicine. EMBO J 33, 409-417. doi:10.1002/embj.201387098
Jacobs, K., Zambelli, F., Mertzanidou, A. et al. (2016). Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Rep 6, 330-341. doi:10.1016/j.stemcr.2016.01.015
Kang, X., Yu, Q., Huang, Y. et al. (2015). Effects of integrating and non-integrating reprogramming methods on copy number variation and genomic stability of human induced pluripotent stem cells. PLoS One 10, e0131128. doi:10.1371/journal.pone.0131128
Kanninen, L. K., Harjumäki, R., Peltoniemi, P. et al. (2016). Laminin-511 and laminin-521-based matrices for efficient hepatic specification of human pluripotent stem cells. Biomaterials 103, 86-100. doi:10.1016/j.biomaterials.2016.06.054
Khan, A. (2020). QDNAseq.hg38: QDNAseq bin annotation for the human genome build hg38. doi:10.5281/ZENODO.4274556
Kibbey, M. C. (1994). Maintenance of the EHS sarcoma and Matrigel preparation. J Tissue Cult Methods 16, 227-230. doi:10.1007/BF01540656
Kilpinen, H., Goncalves, A., Leha, A. et al. (2017). Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 546, 370-375. doi:10.1038/nature22403
Lake, B. B., Chen, S., Hoshi, M. et al. (2019). A single-nucleus RNA-sequencing pipeline to decipher the molecular anatomy and pathophysiology of human kidneys. Nat Commun 10, 2832. doi:10.1038/s41467-019-10861-2
Lam, M. T. and Longaker, M. T. (2012). Comparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med 6, Suppl 3, s80-s86. doi:10.1002/term.1499
Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. https://arxiv.org/abs/1303.3997v2 (accessed 16.12.2021)
Limonciel, A., Ates, G., Carta, G. et al. (2018). Comparison of base-line and chemical-induced transcriptomic responses in HepaRG and RPTEC/TERT1 cells using TempO-Seq. Arch Toxicol 92, 2517-2531. doi:10.1007/s00204-018-2256-2
Lin, M.-H., Miller, J. B., Kikkawa, Y. et al. (2018). Laminin-521 protein therapy for glomerular basement membrane and podocyte abnormalities in a model of Pierson syndrome. J Am Soc Nephrol 29, 1426-1436. doi:10.1681/ASN.2017060690
Liu, P., Kaplan, A., Yuan, B. et al. (2014). Passage number is a major contributor to genomic structural variations in mouse iPSCs. Stem Cells 32, 2657-2667. doi:10.1002/stem.1779
Love, M. I., Huber, W. and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. doi:10.1186/s13059-014-0550-8
Lu, H. F., Chai, C., Lim, T. C. et al. (2014). A defined xeno-free and feeder-free culture system for the derivation, expansion and direct differentiation of transgene-free patient-specific induced pluripotent stem cells. Biomaterials 35, 2816-2826. doi:10.1016/j.biomaterials.2013.12.050
Lu, J., Baccei, A., Lummertz da Rocha, E. et al. (2018). Single-cell RNA sequencing reveals metallothionein heterogeneity during hESC differentiation to definitive endoderm. Stem Cell Res 28, 48-55. doi:10.1016/j.scr.2018.01.015
Lu, Y., Ye, Y., Bao, W. et al. (2017). Genome-wide identification of genes essential for podocyte cytoskeletons based on single-cell RNA sequencing. Kidney Int 92, 1119-1129. doi:10.1016/j.kint.2017.04.022
Meredith, J. E., Winitz, S., Lewis, J. M. A. et al. (1996). The regulation of growth and intracellular signaling by integrins. Endocr Rev 17, 207-220. doi:10.1210/edrv-17-3-207
Morrison, M., Klein, C., Clemann, N. et al. (2015). StemBANCC: Governing access to material and data in a large stem cell research consortium. Stem Cell Rev 5, 681-687. doi:10.1007/s12015-015-9599-3
Murphy, C., Feifel, E., Jennings, P. et al. (2019). A protocol for one-step differentiation of human induced pluripotent stem cells into mature podocytes. Methods Mol Biol 1994, 93-99. doi:10.1007/978-1-4939-9477-9_8
Musah, S., Dimitrakakis, N., Camacho, D. M. et al. (2018). Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat Protoc 13, 1662-1685. doi:10.1038/s41596-018-0007-8
Nagaoka, M., Kobayashi, M., Kawai, C. et al. (2015). Design of a vitronectin-based recombinant protein as a defined substrate for differentiation of human pluripotent stem cells into hepatocyte-like cells. PLoS One 10, e0136350. doi:10.1371/journal.pone.0136350
Nagasaka, R., Matsumoto, M., Okada, M. et al. (2017). Visualization of morphological categories of colonies for monitoring of effect on induced pluripotent stem cell culture status. Regen Ther 6, 41-51. doi:10.1016/j.reth.2016.12.003
Pace, J. A., Bronstein, R., Guo, Y. et al. (2021). Podocyte-specific KLF4 is required to maintain parietal epithelial cell quiescence in the kidney. Sci Adv 7, eabg6600. doi:10.1126/sciadv.abg6600
Padhi, A. and Nain, A. S. (2020). ECM in differentiation: A review of matrix structure, composition and mechanical properties. Ann Biomed Eng 48, 1071-1089. doi:10.1007/s10439-019-02337-7
Pan, G. and Thomson, J. A. (2007). Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res 17, 42-49. doi:10.1038/sj.cr.7310125
Quinlan, A. R. and Hall, I. M. (2010). BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841-842. doi:10.1093/bioinformatics/btq033
Rauch, C., Feifel, E., Kern, G. et al. (2018). Differentiation of human iPSCs into functional podocytes. PLoS One 13, e0203869. doi:10.1371/journal.pone.0203869
Refaeli, I., Hughes, M. R., Wong, A. K.-W. et al. (2020). Distinct functional requirements for podocalyxin in immature and mature podocytes reveal mechanisms of human kidney disease. Sci Rep 10, 9419. doi:10.1038/s41598-020-64907-3
Rinschen, M. M., Huesgen, P. F. and Koch, R. E. (2018). The podocyte protease web: Uncovering the gatekeepers of glomerular disease. Am J Physiol Renal Physiol 315, F1812-F1816. doi:10.1152/ajprenal.00380.2018
Rodin, S., Domogatskaya, A., Ström, S. et al. (2010). Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 28, 611-615. doi:10.1038/nbt.1620
Rodin, S., Antonsson, L., Niaudet, C. et al. (2014). Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun 5, 3195. doi:10.1038/ncomms4195
Rowland, T. J., Miller, L. M., Blaschke, A. J. et al. (2010). Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev 19, 1231-1240. doi:10.1089/scd.2009.0328
Scheinin, I., Sie, D., Bengtsson, H. et al. (2014). DNA copy number analysis of fresh and formalin-fixed specimens by shallow whole-genome sequencing with identification and exclusion of problematic regions in the genome assembly. Genome Res 24, 2022-2032. doi:10.1101/gr.175141.114
Singh, P., Chandrasekaran, V., Hardy, B. et al. (2021). Temporal transcriptomic alterations of cadmium exposed human iPSC-derived renal proximal tubule-like cells. Toxicol In Vitro 76, 105229. doi:10.1016/J.TIV.2021.105229
Slamecka, J., McClellan, S., Wilk, A. et al. (2018). Induced pluripotent stem cells derived from human amnion in chemically defined conditions. Cell Cycle 17, 330-347. doi:10.1080/15384101.2017.1403690
Song, B., Smink, A. M., Jones, C. V. et al. (2012). The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7, e46453. doi:10.1371/journal.pone.0046453
Suter-Dick, L., Alves, P. M., Blaauboer, B. J. et al. (2015). Stem cell-derived systems in toxicology assessment. Stem Cells Dev 24, 1284-1296. doi:10.1089/scd.2014.0540
Suzuki, S., Oldberg, A., Hayman, E. G. et al. (1985). Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J 4, 2519-2524. doi:10.1002/j.1460-2075.1985.tb03965.x
Takahashi, K. and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. doi:10.1016/j.cell.2006.07.024
Theocharis, A. D., Skandalis, S. S., Gialeli, C. et al. (2016). Extracellular matrix structure. Adv Drug Deliv Rev 97, 4-27. doi:10.1016/j.addr.2015.11.001
Tsankov, A. M., Akopian, V., Pop, R. et al. (2015). A qPCR ScoreCard quantifies the differentiation potential of human pluripotent stem cells. Nat Biotechnol 33, 1182-1192. doi:10.1038/nbt.3387
Vierkotten, S., Muether, P. S. and Fauser, S. (2011). Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS One 6, e22959. doi:10.1371/journal.pone.0022959
van de Wiel, M. A., Kim, K. I., Vosse, S. J. et al. (2007). CGHcall: Calling aberrations for array CGH tumor profiles. Bioinformatics 23, 892-894. doi:10.1093/bioinformatics/btm030
Wieser, M., Stadler, G., Jennings, P. et al. (2008). hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295, F1365-F1375. doi:10.1152/ajprenal.90405.2008
Wight, T. N., Kang, I., Evanko, S. P. et al. (2020). Versican – A critical extracellular matrix regulator of immunity and inflammation. Front Immunol 11, 512. doi:10.3389/fimmu.2020.00512
Wilmes, A., Rauch, C., Carta, G. et al. (2017). Towards optimisation of induced pluripotent cell culture: Extracellular acidification results in growth arrest of iPSC prior to nutrient exhaustion. Toxicol In Vitro 45, 445-454. doi:10.1016/j.tiv.2017.07.023
Wolford, J. L., Chishti, Y., Jin, Q. et al. (2010). Loss of pluripotency in human embryonic stem cells directly correlates with an increase in nuclear zinc. PLoS One 5, e12308. doi:10.1371/journal.pone.0012308
Young, J. C., Major, A. T., Miyamoto, Y. et al. (2011). Distinct effects of importin α2 and α4 on Oct3/4 localization and expression in mouse embryonic stem cells. FASEB J 25, 3958-3965. doi:10.1096/fj.10-176941
Zhang, P., Andrianakos, R., Yang, Y. et al. (2010). Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating nanog gene expression. J Biol Chem 285, 9180-9189. doi:10.1074/jbc.M109.077958