Comparison of pyrogen assays by testing products exhibiting low endotoxin recovery
Main Article Content
Abstract
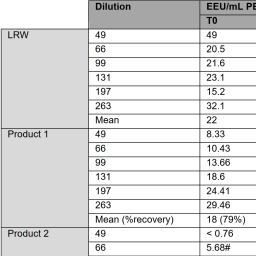
The use of pyrogen tests to assess the risk of endotoxin in biological products has increased recently due to concerns of some regulatory authorities about products exhibiting low endotoxin recovery (LER). Manufacturers increasingly seek to reduce the use of animals unless essential to assure patient safety. The current study compares the ability of the monocyte activation test (MAT) and the bacterial endotoxin test (BET) to the rabbit pyrogen test (RPT) to detect endotoxin spikes in samples of products shown to exhibit LER. Product samples or water were spiked with endotoxin and held for three days or tested immediately in the BET, the RPT, and two variations of the MAT at the same time. Results show high sensitivity to endotoxin of both the BET and MAT, and much lower sensitivity of the RPT, indicating that much higher levels of reference standard endotoxin are required to induce pyrogenicity in the RPT than the 5 endotoxin units (EU) per kg common threshold. The results of the BET and MAT correlated well for the detection of endotoxin spike in water. We also show that LER (masking of endotoxin) found in the BET is also seen in the MAT and RPT, suggesting that the products themselves elicit a biological inactivation of spiked endotoxin over time, thereby rendering it less or non-pyrogenic. We conclude that the non-animal MAT option is a suitable replacement for the RPT to measure spiked endotoxin in biopharmaceuticals.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Bleeker, W. K., de Groot, E. M., den Boer, P. J. et al. (1994). Measurement of interleukin-6 production by monocytes for in vitro safety testing of hemoglobin solutions. Artif Cells Blood Substit Immobil Biotechnol 22, 835-840. doi:10.3109/10731199409117918
Bolden, J., Knutsen, C., Levin, J. et al. (2020). Currently available recombinant alternatives to horseshoe crab blood lysates: Are they comparable for the detection of environmental bacterial endotoxins? A review. PDA J Pharm Sci Technol 74, 602-611. doi:10.5731/pdajpst.2020.012187Chen, D., von Wintzingerode, F., Barlasov-Brown, J. et al. (2019). Low endotoxin recovery. Parenteral Drug Association© Technical Report 82, 1-128. ISBN 9781945584077. https://techpubportal.pda.org
EDQM – European Directorate for the Quality of Medicines and HealthCare (2020a). Chapter 2.6.8 Pyrogens. European Pharmacopoeia. https://pheur.edqm.eu/home
EDQM (2020b). Chapter 2.6.14 Bacterial Endotoxins. European Pharmacopoeia. https://pheur.edqm.eu/home
EDQM (2020c). Chapter 2.6.30 Monocyte Activation Test. European Pharmacopoeia. https://pheur.edqm.eu/home
EDQM (2020d). Chapter 5.1.10 Guidelines for Using the Test for Bacterial Endotoxins. European Pharmacopoeia. https://pheur.edqm.eu/home
EDQM (2021a). Chapter 2.6.32 Test for bacterial endotoxins using recombinant factor C (rFc). European Pharmacopoeia. https://pheur.edqm.eu/home
EDQM (2021b). European Pharmacopoeia to put an end to the rabbit pyrogen test. European Pharmacopoeia Animal Testing News. Strasbourg, France. https://www.edqm.eu/en/news/european-pharmacopoeia-put-end-rabbit-pyrogen-test (accessed 23.05.2022)
Fennrich, S., Fischer, M., Hartung, T. et al. (1999). Detection of endotoxins and other pyrogens using human whole blood. Dev Biol Stand 101, 131-139.
Hartung, T. (2021). Pyrogen testing revisited on the occasion of the 25th anniversary of the whole blood monocyte activation test. ALTEX 38, 3-19. doi:10.14573/altex.2101051
Helle, M., Brakenhoff, J. P., De Groot, E. R. et al. (1988). Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol 18, 957-959. doi:10.1002/eji.1830180619
Hoffman, S., Luderitz-Puchel, U., Montag, T. et al. (2005). Optimisation of pyrogen testing in parenterals according to different pharmacopoeias by probabilistic modelling. J Endotoxin Res 11, 25-31. doi:10.1179/096805105225006678
Kikkert, R., Bulder, I., de Groot, E. R. et al. (2007). Potentiation of Toll-like receptor-induced cytokine production by (1→3)-beta-D-glucans: Implications for the monocyte activation test. J Endotoxin Res 13, 140-149. doi:10.1177/0968051907080024
Kikuchi, H., Hashima, Y., Fukui, C. et al. (2017). Collaborative study on the bacterial endotoxins test using recombinant factor C-based procedure for detection of lipopolysaccharides. Pharm Med Device Regul Sci 48, 252-260.
Molenaar-de Backer, M. W. A., Gitz, E., Dieker, M. et al. (2021). Performance of monocyte activation test supplemented with human serum compared to fetal bovine serum. ALTEX 38, 307-315. doi:10.14573/altex.2008261
Mozier, N. (2019). Chapter 13: Monocyte activation test as a developmental tool for pyrogens. In K. L. Williams (ed.), Endotoxin Detection and Control in Pharma, Limulus and Mammalian Systems (537-545). Springer Nature Switzerland AG. doi:10.1007/978-3-030-17148-3_15
Percie du Sert, N., Hurst, V., Ahluwalia, A. et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Exp Physiol 105, 1459-1466. doi:10.1113/EP088870
Solati, S., Aarden, L., Zeerleder, S. et al. (2015). An improved monocyte activation test using cryopreserved pooled human mononuclear cells. Innate Immun 21, 677-684. doi:10.1177/1753425915583365
Tindall, B., Demircioglu, D. and Uhlig, T. (2021). Recombinant bacterial endotoxin testing: A proven solution. BioTechniques 70, 290-300. doi:10.2144/btn-2020-0165
US FDA (2012) Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers (accessed 23.05.2022)
USP – United States Pharmacopeia (2022a). General Chapter <85>. Bacterial Endotoxins Test. USP-NF. Rockville, MD, USA: United States Pharmacopeia. doi:10.31003/USPNF_M98830_02_01
USP (2022b). General Chapter <151> Pyrogen Test. USP-NF. Rockville, MD: United States Pharmacopeia. doi:10.31003/USPNF_M98900_01_01