State-of-the-art and new options to assess T cell activation by skin sensitizers: Cosmetics Europe Workshop
Main Article Content
Abstract
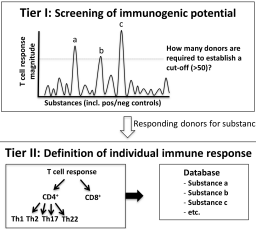
Significant progress has been made in the development and validation of non-animal test methods for skin sensitization assessment. At present, three of the four key events of the adverse outcome pathway (AOP) are assessable by OECD-accepted in vitro methods. The fourth key event describes the immunological response in the draining lymph node where activated dendritic cells present major histocompatibility complex-bound chemically modified peptides to naïve T cells, thereby priming the proliferation of antigen-specific T cells. Despite substantial efforts, modelling and assessing this adaptive immune response to sensitizers with in vitro T cell assays still represents a challenge. The Cosmetics Europe Skin Tolerance Task Force organized a workshop, bringing together academic researchers, method developers, industry representatives and regulatory stakeholders to review the scientific status of T cell-based assays, foster a mutual scientific understanding and conceive new options to assess T cell activation. Participants agreed that current T cell assays have come a long way in predicting immunogenicity, but that further investment and collaboration is required to simplify assays, optimize their sensitivity, better define human donor-to-donor variability and evaluate their value to predict sensitizer potency. Furthermore, the potential role of T cell assays in AOP-based testing strategies and subsequent safety assessment concepts for cosmetic ingredients was discussed. It was agreed that it is currently difficult to anticipate uses of T cell assay data for safety assessment and concluded that experience from case studies on real-life risk assessment scenarios is needed to further consider the usefulness of assessing the fourth AOP key event.
Article Details
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).