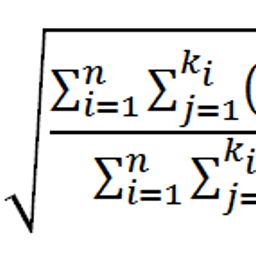Dear readers,
we are very pleased to announce the winner of the ALTEX Prize 2019: Fabian Grimm, formerly of the Texas A&M University, now at ExxonMobil Biomedical Sciences, Inc. will be awarded the prize for the best manuscript published in ALTEX during 2018 at the social event of the EUSAAT Congress this October. Congratulations!
We are thrilled that the impact factor of ALTEX has increased further to 6.183. A warm thank you to all scientists who entrust the journal with their manuscripts, to the reviewers who invest their time to appraise the manuscripts and share their critical thoughts and questions with the authors, and to our staff, who shape and polish the texts and figures into articles. It is encouraging to witness the increasing recognition of work on alternatives to animal experiments by the scientific community.
2019 marks the 60th anniversary of the 3Rs, first proposed in the Principles of Humane Experimental Technique. This is an excellent time to take a look at how far the field has come and to think about what could be done to more effectively protect animals given the new tools and models that are now becoming available. Kathrin Herrmann, Francesca Pistollato, and our North American editor Marty Stephens provide Food for Thought … on a strategy to step into the next decade.
An adverse outcome pathway (AOP) describes the chain of events leading from an initial trigger to a health issue. Christy Foran and colleagues present a way to model how much deflection of one element will set off the subsequent element of the chain in order to build a quantitative AOP that can predict the relative level of an adverse effect different substances will have on a system.
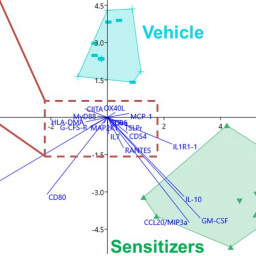
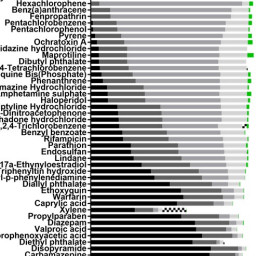
Haojian Li et al. alert us to the issue that prediction models are often based on data from relatively few substances of which usually more are positive than negative. Using the large Cosmetics Europe database, they have compared data-rebalancing ensemble learning models to improve the predictivity of defined approaches for skin sensitization assessment.
Some skin sensitizers only become active allergens upon exposure to light. Nitin Patel and colleagues demonstrate that modifications of in chemico skin sensitization assays that include UV irradiation identify known photosensitizers based on their ability to bind model peptides representing the haptenization process in the skin.
Two manuscripts present work on different versions of the same multi-cell type 3D alveolar model. Diego Marescotti et al. show the contributions of each of four cell types to the functionality of the model using biological network models. They compare the transcriptional profile of their model to that of other in vitro airway models and human lung tissue and show that complex model systems built from multiple cell types in a more physiological state closely approach the profile of normal human lung tissue. Aline Chary et al. develop a modification of the model involving also dendritic-like cells to discriminate between respiratory sensitizers and irritants. They identify differences in cytokine release and surface marker expression that allow identification of low and high molecular weight respiratory sensitizers.
Fatty tissue is more than an energy store. Outi Huttala and colleagues show that allowing vascularization of adipose tissue by culturing adipocytes together with human umbilical cord vein endothelial cells allows it to respond faster to insulin and modulates its response to other angiogenic or adipogenic chemicals in comparison to the same model in the absence of vasculature. This well characterized, complex in vitro model provides the basis to study the functionality of human adipose tissue without the use of animals.
In developing a test for a biological activity, one naturally aims to find a readout and define a cut-off that clearly discriminates between positive and negative substances. However, the reality is that some substances are falsely identified as positive or negative, respectively. Using data from skin sensitization testing, Maria Leontaridou and colleagues show that evaluation of a test’s predictivity is more informative and allows a better comparison with other tests if it includes a description of the variability and the upper and lower limits.
Challenging cells with a chemical seems like one of the simpler parts of a test until you start to ask how much of the chemical you add actually reaches the cells and how this is influenced by the composition of the medium, the incubation vessel, and the chemical’s own properties. Susana Proença et al. model the biokinetics of substances in in vitro tests to determine the concentrations that are available to the cells under different conditions to allow comparison of results from different tests.
A short communication by George Helman and colleagues introduces a read-across workflow on the EPA CompTox Chemicals Dashboard, and the two letters share experience of the “Valley of Death” between developing and actually implementing an alternative method and ask how often applications for animal experiments are actually rejected in Germany.
The BenchMarks series continues with two contributions: Stevie van der Mierden and colleagues explore literature screening tools for systematic reviews and Christian Karreman et al. introduce an image analysis application they have developed to quantify colocalized fluorescence signals of overlapping cells.
Four Meeting Reports and six Corners bring you up to date on recent and upcoming activities in the 3Rs field; you can find news and a calendar of upcoming events at https://www.altex.org.
Enjoy this issue of ALTEX and see you in Linz in October.
Sonja von Aulock
Editor in chief, ALTEX
Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research
https://doi.org/10.14573/altex.1907031
A modular approach for assembly of quantitative adverse outcome pathways
https://doi.org/10.14573/altex.1810181
Improved defined approaches for predicting skin sensitization hazard and potency in humans
https://doi.org/10.14573/altex.1809191
An in vitro coculture system for the detection of sensitization following aerosol exposure
https://doi.org/10.14573/altex.1901241
The impact of precision uncertainty on predictive accuracy metrics of non-animal testing methods
https://doi.org/10.14573/altex.1810111
Insights into in vitro biokinetics using Virtual Cell Based Assay simulations
https://doi.org/10.14573/altex.1812101
Generalized Read-Across (GenRA): A workflow implemented into the EPA CompTox Chemicals Dashboard
https://doi.org/10.14573/altex.1811292
Applications for animal experiments are rarely rejected in Germany
https://doi.org/10.14573/altex.1906111
Implementing Data – The Future of Alternative Methods – VZET Symposium 2019
https://doi.org/10.14573/altex.1906211
The biological evaluation of medical devices: Transition to 2017/745 MDR in progress
https://doi.org/10.14573/altex.1907011
Building blocks for a European Organ-on-Chip roadmap
https://doi.org/10.14573/altex.1905221
Corrigendum to Normalization of data for viability and relative cell function curves
https://doi.org/10.14573/altex.1904113
Corrigendum to Essential components of methods papers
https://doi.org/10.14573/altex.1904114
Software tools for literature screening in systematic reviews in biomedical research
https://doi.org/10.14573/altex.1902131
SUIKER: Quantification of antigens in cell organelles, neurites and cellular sub-structures by imaging
https://doi.org/10.14573/altex.1906251