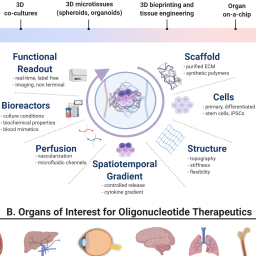
Leveraging microphysiological systems to address challenges encountered during development of oligonucleotide therapeutics
Main Article Content
Abstract
Oligonucleotide therapeutics (ONTs) encompass classes of medicines that selectively target and potentially ameliorate previously untreatable and often rare diseases. Several unique classes of ONTs provide versatility, enabling direct modulation of gene expression by virtue of Watson-Crick base pairing or modulation of cell signaling through structural mimicry or interference with protein-receptor interactions. Due to a lack of suitable in vitro models capable of recapitulating or predicting in vivo effects of ONTs, their discovery and optimization has relied heavily on animal studies for predicting efficacy and safety in humans. Since ONTs often lack cross-species activity, animal models with genetic humanization and/or species-specific surrogate ONTs are often required. Human microphysiological systems (MPS) offer an opportunity to reduce the use of animals and may enable evaluation of drug mechanisms, optimization of cell and tissue targeting ligands or delivery vehicles, and characterization of pharmacokinetics (PK), pharmacodynamics (PD), and safety of candidate ONTs. The lack of published examples for MPS applications with ONT demonstrates the need for a focused effort to characterize and build confidence in their utility. The goals of this review are to summarize the current landscape of ONTs and highlight potential opportunities and challenges for application of MPS during ONT discovery and development. In addition, this review aims to raise awareness with ONT drug developers and regulatory authorities on the potential impact of MPS with respect to characterizing pharmacology, ADME, and toxicity and to educate MPS platform developers on unique design attributes needed to fully appreciate MPS advantages in ONT development.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Adami, R. and Bottai, D. (2019). Spinal muscular atrophy modeling and treatment advances by induced pluripotent stem cells studies. Stem Cell Rev Rep 15, 795-813. doi:10.1007/s12015-019-09910-6
Agarwal, S., Simon, A. R., Goel, V. et al. (2020). Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin Pharmacol Ther 108, 63-72. doi:10.1002/cpt.1802
Ainslie, G. R., Davis, M., Ewart, L. et al. (2019). Microphysiological lung models to evaluate the safety of new pharmaceutical modalities: A biopharmaceutical perspective. Lab Chip 19, 3152-3161. doi:10.1039/c9lc00492k
Alberer, M., Gnad-Vogt, U., Hong, H. S. et al. (2017). Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390, 1511-1520. doi:10.1016/S0140-6736(17)31665-3
Alton, E. W., Boushey, H. A., Garn, H. et al. (2012). Clinical expert panel on monitoring potential lung toxicity of inhaled oligonucleotides: Consensus points and recommendations. Nucleic Acid Ther 22, 246-254. doi:10.1089/nat.2012.0345
Ananthakumar, A., Liu, Y., Fernandez, C. E. et al. (2020). Modeling statin myopathy in a human skeletal muscle microphysiological system. PLoS One 15, e0242422. doi:10.1371/journal.pone.0242422
Andersson, P. and den Besten, C. (2019). Preclinical and clinical drug-metabolism, pharmacokinetics and safety of therapeutic oligonucleotides. Chapter 20. In S. Agrawal and M. J. Gait (eds.), Advances in Nucleic Acid Therapeutics. The Royal Society of Chemistry. doi:10.1039/9781788015714-00474
Avery, R. L., Castellarin, A. A., Steinle, N. C. et al. (2014). Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98, 1636-1641. doi:10.1136/bjophthalmol-2014-305252
Bai, J. and Wang, C. (2020). Organoids and microphysiological systems: New tools for ophthalmic drug discovery. Front Pharmacol 11, 407. doi:10.3389/fphar.2020.00407
Bajaj, P., Chowdhury, S. K., Yucha, R. et al. (2018). Emerging kidney models to investigate metabolism, transport, and toxicity of drugs and xenobiotics. Drug Metab Dispos 46, 1692-1702. doi:10.1124/dmd.118.082958
Barrile, R., van der Meer, A. D., Park, H. et al. (2018). Organ-on-chip recapitulates thrombosis induced by an anti-CD154 monoclonal antibody: Translational potential of advanced microengineered systems. Clin Pharmacol Ther 104, 1240-1248. doi:10.1002/cpt.1054
Baudy, A. R., Otieno, M. A., Hewitt, P. et al. (2020). Liver microphysiological systems development guidelines for safety risk assessment in the pharmaceutical industry. Lab Chip 20, 215-225. doi:10.1039/c9lc00768g
Bell, C. C., Hendriks, D. F., Moro, S. M. et al. (2016). Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep 6, 25187. doi:10.1038/srep25187
Bell, C. C., Dankers, A. C. A., Lauschke, V. M. et al. (2018). Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: A multicenter study. Toxicol Sci 162, 655-666. doi:10.1093/toxsci/kfx289
Berman, C. L., Cannon, K., Cui, Y. et al. (2014). Recommendations for safety pharmacology evaluations of oligonucleotide-based therapeutics. Nucleic Acid Ther 24, 291-301. doi:10.1089/nat.2013.0477
Berman, C. L., Barros, S. A., Galloway, S. M. et al. (2016). OSWG recommendations for genotoxicity testing of novel oligonucleotide-based therapeutics. Nucleic Acid Ther 26, 73-85. doi:10.1089/nat.2015.0534
Biliouris, K., Gaitonde, P., Yin, W. et al. (2018). A semi-mechanistic population pharmacokinetic model of nusinersen: An antisense oligonucleotide for the treatment of spinal muscular atrophy. CPT Pharmacometrics Syst Pharmacol 7, 581-592. doi:10.1002/psp4.12323
Blin, A., Le Goff, A., Magniez, A. et al. (2016). Microfluidic model of the platelet-generating organ: Beyond bone marrow biomimetics. Sci Rep 6, 21700. doi:10.1038/srep21700
Brown, C. R., Gupta, S., Qin, J. et al. (2020). Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res 48, 11827-11844. doi:10.1093/nar/gkaa670
Brown, J. A., Codreanu, S. G., Shi, M. et al. (2016). Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J Neuroinflammation 13, 306. doi:10.1186/s12974-016-0760-y
Buntz, A., Killian, T., Schmid, D. et al. (2019). Quantitative fluorescence imaging determines the absolute number of locked nucleic acid oligonucleotides needed for suppression of target gene expression. Nucleic Acids Res 47, 953-969. doi:10.1093/nar/gky1158
Capaldi, D., Teasdale, A., Henry, S. et al. (2017). Impurities in oligonucleotide drug substances and drug products. Nucleic Acid Ther 27, 309-322. doi:10.1089/nat.2017.0691
Capowski, E. E., Samimi, K., Mayerl, S. J. et al. (2019). Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146, dev171686. doi:10.1242/dev.171686
Castellanos-Rizaldos, E., Brown, C. R., Dennin, S. et al. (2020). RT-qPCR methods to support pharmacokinetics and drug mechanism of action to advance development of RNAi therapeutics. Nucleic Acid Ther 30, 133-142. doi:10.1089/nat.2019.0840
Cavagnaro, J., Berman, C., Kornbrust, D. et al. (2014). Considerations for assessment of reproductive and developmental toxicity of oligonucleotide-based therapeutics. Nucleic Acid Ther 24, 313-325. doi:10.1089/nat.2014.0490
Chi, K. N., Higano, C. S., Blumenstein, B. et al. (2017). Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): A phase 3, multicentre, open-label, randomised trial. Lancet Oncol 18, 473-485. doi:10.1016/S1470-2045(17)30168-7
Claborn, M. K., Stevens, D. L., Walker, C. K. et al. (2019). Nusinersen: A treatment for spinal muscular atrophy. Ann Pharmacother 53, 61-69. doi:10.1177/1060028018789956
Crooke, S. T., Baker, B. F., Witztum, J. L. et al. (2017a). The effects of 2'-o-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther 27, 121-129. doi:10.1089/nat.2016.0650
Crooke, S. T., Wang, S., Vickers, T. A. et al. (2017b). Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol 35, 230-237. doi:10.1038/nbt.3779
Crooke, S. T., Liang, X. H., Crooke, R. M. et al. (2020a). Antisense drug discovery and development technology considered in a pharmacological context. Biochem Pharmacol 189, 114196. doi:10.1016/j.bcp.2020.114196
Crooke, S. T., Vickers, T. A. and Liang, X. H. (2020b). Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res 48, 5235-5253. doi:10.1093/nar/gkaa299
Crooke, S. T., Liang, X. H., Baker, B. F. et al. (2021). Antisense technology: A review. J Biol Chem 296, 100416. doi:10.1016/j.jbc.2021.100416
Csobonyeiova, M., Polak, S. and Danisovic, L. (2020). Recent overview of the use of iPSCs Huntington’s disease modeling and therapy. Int J Mol Sci 21, 2239. doi:10.3390/ijms21062239
de Fougerolles, A., Vornlocher, H. P., Maraganore, J. et al. (2007). Interfering with disease: A progress report on siRNA-based therapeutics. Nat Rev Drug Discov 6, 443-453. doi:10.1038/nrd2310
Debacker, A. J., Voutila, J., Catley, M. et al. (2020). Delivery of oligonucleotides to the liver with GalNAc: From research to registered therapeutic drug. Mol Ther 28, 1759-1771. doi:10.1016/j.ymthe.2020.06.015
Dembska, A., Switalska, A., Fedoruk-Wyszomirska, A. et al. (2020). Development of fluorescence oligonucleotide probes based on cytosine- and guanine-rich sequences. Sci Rep 10, 11006. doi:10.1038/s41598-020-67745-5
Dirin, M. and Winkler, J. (2013). Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin Biol Ther 13, 875-888. doi:10.1517/14712598.2013.774366
Echigoya, Y., Lim, K. R. Q., Trieu, N. et al. (2017). Quantitative antisense screening and optimization for exon 51 skipping in duchenne muscular dystrophy. Mol Ther 25, 2561-2572. doi:10.1016/j.ymthe.2017.07.014
EMA – European Medicines Agency (2011). ICH guideline S6 (R1) – preclinical safety evaluation of biotechnology-derived pharmaceuticals. https://www.ema.europa.eu/documents/scientific-guideline/ich-s6r1-preclinical-safety-evaluation-biotechnology-derived-pharmaceuticals-step-5_en.pdf
Fabre, K., Berridge, B., Proctor, W. R. et al. (2020). Introduction to a manuscript series on the characterization and use of microphysiological systems (MPS) in pharmaceutical safety and ADME applications. Lab Chip 20, 1049-1057. doi:10.1039/c9lc01168d
FDA – Food and Drug Administration, HHS (2010). International conference on harmonisation; guidance on M3(R2) nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. Fed Regist 75, 3471-3472.
Feldman, R. A., Fuhr, R., Smolenov, I. et al. (2019). mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37, 3326-3334. doi:10.1016/j.vaccine.2019.04.074
Felekkis, K., Touvana, E., Stefanou, C. et al. (2010). microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 14, 236-240.
Foster, D. J., Brown, C. R., Shaikh, S. et al. (2018). Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther 26, 708-717. doi:10.1016/j.ymthe.2017.12.021
Frazier, K. S. (2015). Antisense oligonucleotide therapies: The promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol 43, 78-89. doi:10.1177/0192623314551840
Frazier, K. S. and Obert, L. A. (2018). Drug-induced glomerulonephritis: The spectre of biotherapeutic and antisense oligonucleotide immune activation in the kidney. Toxicol Pathol 46, 904-917. doi:10.1177/0192623318789399
Fuchs, S., Johansson, S., Tjell, A. O. et al. (2021). In-line analysis of organ-on-chip systems with sensors: Integration, fabrication, challenges, and potential. ACS Biomater Sci Eng 7, 2926-2948. doi:10.1021/acsbiomaterials.0c01110
Geary, R. S., Norris, D., Yu, R. et al. (2015). Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87, 46-51. doi:10.1016/j.addr.2015.01.008
Gholobova, D., Gerard, M., Decroix, L. et al. (2018). Human tissue-engineered skeletal muscle: A novel 3D in vitro model for drug disposition and toxicity after intramuscular injection. Sci Rep 8, 12206. doi:10.1038/s41598-018-30123-3
Giadone, R. M., Rosarda, J. D., Akepati, P. R. et al. (2018). A library of ATTR amyloidosis patient-specific induced pluripotent stem cells for disease modelling and in vitro testing of novel therapeutics. Amyloid 25, 148-155. doi:10.1080/13506129.2018.1489228
Godinho, B., Gilbert, J. W., Haraszti, R. A. et al. (2017). Pharmacokinetic profiling of conjugated therapeutic oligonucleotides: A high-throughput method based upon serial blood microsampling coupled to peptide nucleic acid hybridization assay. Nucleic Acid Ther 27, 323-334. doi:10.1089/nat.2017.0690
Goemans, N., Mercuri, E., Belousova, E. et al. (2018). A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in duchenne muscular dystrophy. Neuromuscul Disord 28, 4-15. doi:10.1016/j.nmd.2017.10.004
Graham, M. J., Crooke, S. T., Monteith, D. K. et al. (1998). In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration. J Pharmacol Exp Ther 286, 447-458.
Groell, F., Jordan, O. and Borchard, G. (2018). In vitro models for immunogenicity prediction of therapeutic proteins. Eur J Pharm Biopharm 130, 128-142. doi:10.1016/j.ejpb.2018.06.008
Habtemariam, B. A., Karsten, V., Attarwala, H. et al. (2020). Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther 109, 372-382. doi:10.1002/cpt.1974
Hanagata, N. (2017). CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int J Nanomedicine 12, 515-531. doi:10.2147/IJN.S114477
Haque, A., Gheibi, P., Gao, Y. et al. (2016). Cell biology is different in small volumes: Endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci Rep 6, 33980. doi:10.1038/srep33980
Hardwick, R. N., Betts, C. J., Whritenour, J. et al. (2020). Drug-induced skin toxicity: Gaps in preclinical testing cascade as opportunities for complex in vitro models and assays. Lab Chip 20, 199-214. doi:10.1039/c9lc00519f
Haring, A. P., Sontheimer, H. and Johnson, B. N. (2017). Microphysiological human brain and neural systems-on-a-chip: Potential alternatives to small animal models and emerging platforms for drug discovery and personalized medicine. Stem Cell Rev Rep 13, 381-406. doi:10.1007/s12015-017-9738-0
Henry, S. P., Seguin, R., Cavagnaro, J. et al. (2016). Considerations for the characterization and interpretation of results related to alternative complement activation in monkeys associated with oligonucleotide-based therapeutics. Nucleic Acid Ther 26, 210-215. doi:10.1089/nat.2015.0593
Henry, S. P., Narayanan, P., Shen, L. et al. (2017). Assessment of the effects of 2'-methoxyethyl antisense oligonucleotides on platelet count in cynomolgus nonhuman primates. Nucleic Acid Ther 27, 197-208. doi:10.1089/nat.2017.0666
Hirabayashi, Y., Maki, K., Kinoshita, K. et al. (2021). Considerations of the Japanese research working group for the ICH S6 & related issues regarding nonclinical safety assessments of oligonucleotide therapeutics: Comparison with those of biopharmaceuticals. Nucleic Acid Ther 31, 114-125. doi:10.1089/nat.2020.0879
Hor, J. H., Soh, E. S., Tan, L. Y. et al. (2018). Cell cycle inhibitors protect motor neurons in an organoid model of spinal muscular atrophy. Cell Death Dis 9, 1100. doi:10.1038/s41419-018-1081-0
Jackson, N. A. C., Kester, K. E., Casimiro, D. et al. (2020). The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 5, 11. doi:10.1038/s41541-020-0159-8
Janas, M. M., Schlegel, M. K., Harbison, C. E. et al. (2018). Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat Commun 9, 723. doi:10.1038/s41467-018-02989-4
Janssen, M. J., Nieskens, T. T. G., Steevels, T. A. M. et al. (2019). Therapy with 2'-O-me phosphorothioate antisense oligonucleotides causes reversible proteinuria by inhibiting renal protein reabsorption. Mol Ther Nucleic Acids 18, 298-307. doi:10.1016/j.omtn.2019.08.025
Juliano, R. L. (2016). The delivery of therapeutic oligonucleotides. Nucleic Acids Res 44, 6518-6548. doi:10.1093/nar/gkw236
Keefe, A. D., Pai, S. and Ellington, A. (2010). Aptamers as therapeutics. Nat Rev Drug Discov 9, 537-550. doi:10.1038/nrd3141
Kim, J., Basiri, B., Hassan, C. et al. (2019a). Metabolite profiling of the antisense oligonucleotide eluforsen using liquid chromatography-mass spectrometry. Mol Ther Nucleic Acids 17, 714-725. doi:10.1016/j.omtn.2019.07.006
Kim, Y., Jo, M., Schmidt, J. et al. (2019b). Enhanced potency of GalNAc-conjugated antisense oligonucleotides in hepatocellular cancer models. Mol Ther 27, 1547-1557. doi:10.1016/j.ymthe.2019.06.009
Kock, K. and Brouwer, K. L. (2012). A perspective on efflux transport proteins in the liver. Clin Pharmacol Ther 92, 599-612. doi:10.1038/clpt.2012.79
Koller, E., Vincent, T. M., Chappell, A. et al. (2011). Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res 39, 4795-4807. doi:10.1093/nar/gkr089
Kornbrust, D., Cavagnaro, J., Levin, A. et al. (2013). Oligo safety working group exaggerated pharmacology subcommittee consensus document. Nucleic Acid Ther 23, 21-28. doi:10.1089/nat.2012.0399
Lee, C. S. and Leong, K. W. (2020). Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr Opin Biotechnol 66, 78-87. doi:10.1016/j.copbio.2020.06.009
Lee, S., Kim, S., Koo, D. J. et al. (2021). 3D microfluidic platform and tumor vascular mapping for evaluating anti-angiogenic RNAi-based nanomedicine. ACS Nano 15, 338-350. doi:10.1021/acsnano.0c05110
Li, G., Gao, G., Wang, P. et al. (2019). Generation and characterization of induced pluripotent stem cells and retinal organoids from a Leber’s congenital amaurosis patient with novel rpe65 mutations. Front Mol Neurosci 12, 212. doi:10.3389/fnmol.2019.00212
Lidberg, K. A., Annalora, A. J., Jozic, M. et al. (2021). Antisense oligonucleotide development for the selective modulation of CYP3A5 in renal disease. Sci Rep 11, 4722. doi:10.1038/s41598-021-84194-w
Lin, P. J. and Tam, Y. K. (2015). Enhancing the pharmacokinetic/pharmacodynamic properties of therapeutic nucleotides using lipid nanoparticle systems. Future Med Chem 7, 1751-1769. doi:10.4155/fmc.15.108
Lindow, M., Vornlocher, H. P., Riley, D. et al. (2012). Assessing unintended hybridization-induced biological effects of oligonucleotides. Nat Biotechnol 30, 920-923. doi:10.1038/nbt.2376
Liu, J., Li, J., Tran, C. et al. (2019). Oligonucleotide quantification and metabolite profiling by high-resolution and accurate mass spectrometry. Bioanalysis 11, 1967-1980. doi:10.4155/bio-2019-0137
Lubbers, R., van Essen, M. F., van Kooten, C. et al. (2017). Production of complement components by cells of the immune system. Clin Exp Immunol 188, 183-194. doi:10.1111/cei.12952
Maepa, S. W. and Ndlovu, H. (2020). Advances in generating liver cells from pluripotent stem cells as a tool for modeling liver diseases. Stem Cells 38, 606-612. doi:10.1002/stem.3154
Mancio-Silva, L., Fleming, H. E., Miller, A. B. et al. (2019). Improving drug discovery by nucleic acid delivery in engineered human microlivers. Cell Metab 29, 727-735 e723. doi:10.1016/j.cmet.2019.02.003
Manning, J. and O’Malley, D. (2015). What has the mdx mouse model of duchenne muscular dystrophy contributed to our understanding of this disease? J Muscle Res Cell Motil 36, 155-167. doi:10.1007/s10974-015-9406-4
Marlowe, J. L., Akopian, V., Karmali, P. et al. (2017). Recommendations of the oligonucleotide safety working group’s formulated oligonucleotide subcommittee for the safety assessment of formulated oligonucleotide-based therapeutics. Nucleic Acid Ther 27, 183-196. doi:10.1089/nat.2017.0671
McGreevy, J. W., Hakim, C. H., McIntosh, M. A. et al. (2015). Animal models of Duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis Model Mech 8, 195-213. doi:10.1242/dmm.018424
Messner, S., Fredriksson, L., Lauschke, V. M. et al. (2018). Transcriptomic, proteomic, and functional long-term characterization of multicellular three-dimensional human liver microtissues. Appl In Vitro Toxicol 4, 1-12. doi:10.1089/aivt.2017.0022
Miller, C. P., Shin, W., Ahn, E. H. et al. (2020). Engineering microphysiological immune system responses on chips. Trends Biotechnol 38, 857-872. doi:10.1016/j.tibtech.2020.01.003
Monani, U. R., Sendtner, M., Coovert, D. D. et al. (2000). The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in SMN-/- mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet 9, 333-339. doi:10.1093/hmg/9.3.333
Nagashima, T., Hadiwidjaja, S., Ohsumi, S. et al. (2020). In vitro model of human skeletal muscle tissues with contractility fabricated by immortalized human myogenic cells. Adv Biosyst 4, e2000121. doi:10.1002/adbi.202000121
Nair, J. K., Attarwala, H., Sehgal, A. et al. (2017). Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res 45, 10969-10977. doi:10.1093/nar/gkx818
Narayanan, P., Shen, L., Curtis, B. R. et al. (2018). Investigation into the mechanism(s) that leads to platelet decreases in cynomolgus monkeys during administration of ISIS 104838, a 2'-MOE-modified antisense oligonucleotide. Toxicol Sci 164, 613-626. doi:10.1093/toxsci/kfy119
Nieskens, T. T. G., Magnusson, O., Andersson, P. et al. (2021). Nephrotoxic antisense oligonucleotide SPC5001 induces kidney injury biomarkers in a proximal tubule-on-a-chip. Arch Toxicol 95, 2123-2136. doi:10.1007/s00204-021-03062-8
Norris, D. A., Post, N., Yu, R. Z. et al. (2019). Bioanalysis considerations on the pharmacokinetic evaluation of antisense therapeutics. Bioanalysis 11, 1909-1912. doi:10.4155/bio-2019-0194
OECD (2018). Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation assays addressing the Key Event on activation of dendritic cells on the Adverse Outcome Pathway for Skin Sensitisation. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264264359-en
OECD (2019). Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264264618-en
OECD (2021). Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. doi:10.1787/9789264242845-en
Ohuchi, K., Funato, M., Kato, Z. et al. (2016). Established stem cell model of spinal muscular atrophy is applicable in the evaluation of the efficacy of thyrotropin-releasing hormone analog. Stem Cells Transl Med 5, 152-163. doi:10.5966/sctm.2015-0059
Osaki, T., Serrano, J. C. and Kamm, R. D. (2018). Cooperative effects of vascular angiogenesis and lymphangiogenesis. Regen Eng Transl Med 4, 120-132. doi:10.1007/s40883-018-0054-2
Pardi, N., Hogan, M. J., Porter, F. W. et al. (2018). mRNA vaccines – A new era in vaccinology. Nat Rev Drug Discov 17, 261-279. doi:10.1038/nrd.2017.243
Paz-Ares, L., Douillard, J. Y., Koralewski, P. et al. (2006). Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 24, 1428-1434. doi:10.1200/JCO.2005.04.3299
Pei, Y., Hancock, P. J., Zhang, H. et al. (2010). Quantitative evaluation of siRNA delivery in vivo. RNA 16, 2553-2563. doi:10.1261/rna.2255810
Pellegrini, L., Bonfio, C., Chadwick, J. et al. (2020). Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369, eaaz5626. doi:10.1126/science.aaz5626
Peters, M. F., Choy, A. L., Pin, C. et al. (2020). Developing in vitro assays to transform gastrointestinal safety assessment: Potential for microphysiological systems. Lab Chip 20, 1177-1190. doi:10.1039/c9lc01107b
Peterson, N. C., Mahalingaiah, P. K., Fullerton, A. et al. (2020). Application of microphysiological systems in biopharmaceutical research and development. Lab Chip 20, 697-708. doi:10.1039/c9lc00962k
Phillips, J. A., Grandhi, T. S. P., Davis, M. et al. (2020). A pharmaceutical industry perspective on microphysiological kidney systems for evaluation of safety for new therapies. Lab Chip 20, 468-476. doi:10.1039/c9lc00925f
Ramsden, D., Zhou, J. and Tweedie, D. J. (2015). Determination of a degradation constant for CYP3A4 by direct suppression of mRNA in a novel human hepatocyte model, HepatoPac. Drug Metab Dispos 43, 1307-1315. doi:10.1124/dmd.115.065326
Ramsden, D., Wu, J. T., Zerler, B. et al. (2019). In vitro drug-drug interaction evaluation of GalNAc conjugated siRNAs against CYP450 enzymes and transporters. Drug Metab Dispos 47, 1183-1194. doi:10.1124/dmd.119.087098
Ray, K. K., Wright, R. S., Kallend, D. et al. (2020). Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 382, 1507-1519. doi:10.1056/NEJMoa1912387
Riahi, R., Shaegh, S. A., Ghaderi, M. et al. (2016). Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci Rep 6, 24598. doi:10.1038/srep24598
Ribeiro, A. J. S., Yang, X., Patel, V. et al. (2019). Liver microphysiological systems for predicting and evaluating drug effects. Clin Pharmacol Ther 106, 139-147. doi:10.1002/cpt.1458
Ryvniak, V. V. (1987). Exocytosis of lysosomal enzymes by hepatocytes into the bile during the involutional development of liver cirrhosis [Article in Russian]. Biull Eksp Biol Med 104, 749-750.
Sakolish, C., Chen, Z., Dalaijamts, C. et al. (2020). Predicting tubular reabsorption with a human kidney proximal tubule tissue-on-a-chip and physiologically-based modeling. Toxicol In Vitro 63, 104752. doi:10.1016/j.tiv.2019.104752
Sands, B. E., Feagan, B. G., Sandborn, W. J. et al. (2020). Mongersen (GED-0301) for active Crohn’s disease: Results of a phase 3 study. Am J Gastroenterol 115, 738-745. doi:10.14309/ajg.0000000000000493
Santos Rosalem, G., Gonzales Torres, L. A., de Las Casas, E. B. et al. (2020). Microfluidics and organ-on-a-chip technologies: A systematic review of the methods used to mimic bone marrow. PLoS One 15, e0243840. doi:10.1371/journal.pone.0243840
Schubert, D., Levin, A. A., Kornbrust, D. et al. (2012). The oligonucleotide safety working group (OSWG). Nucleic Acid Ther 22, 211-212. doi:10.1089/nat.2012.0383
Sewing, S., Boess, F., Moisan, A. et al. (2016). Establishment of a predictive in vitro assay for assessment of the hepatotoxic potential of oligonucleotide drugs. PLoS One 11, e0159431. doi:10.1371/journal.pone.0159431
Sewing, S., Roth, A. B., Winter, M. et al. (2017). Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. PLoS One 12, e0187574. doi:10.1371/journal.pone.0187574
Sfriso, R., Zhang, S., Bichsel, C. A. et al. (2018). 3D artificial round section micro-vessels to investigate endothelial cells under physiological flow conditions. Sci Rep 8, 5898. doi:10.1038/s41598-018-24273-7
Sheehan, J. P. and Phan, T. M. (2001). Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex by an allosteric mechanism. Biochemistry 40, 4980-4989. doi:10.1021/bi002396x
Shemesh, C. S., Yu, R. Z., Gaus, H. J. et al. (2016). Elucidation of the biotransformation pathways of a GalNAc3-conjugated antisense oligonucleotide in rats and monkeys. Mol Ther Nucleic Acids 5, e319. doi:10.1038/mtna.2016.31
Shemesh, C. S., Yu, R. Z., Warren, M. S. et al. (2017). Assessment of the drug interaction potential of unconjugated and GalNAc3-conjugated 2'-MOE-ASOs. Mol Ther Nucleic Acids 9, 34-47. doi:10.1016/j.omtn.2017.08.012
Slingsby, M. H. L., Vijey, P., Tsai, I. T. et al. (2021). Sequence-specific 2'-O-methoxyethyl antisense oligonucleotides activate human platelets through glycoprotein VI, triggering formation of platelet-leukocyte aggregates. Haematologica. doi:10.3324/haematol.2020.260059
Sousa, M. M., Fernandes, R., Palha, J. A. et al. (2002). Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol 161, 1935-1948. doi:10.1016/S0002-9440(10)64469-0
Sutherland, J. E., Hettinger, J. L., Chan, A. et al. (2020). Nonclinical safety profile of revusiran, a 1st-generation GalNAc-siRNA conjugate for treatment of hereditary transthyretin-mediated amyloidosis. Nucleic Acid Ther 30, 33-49. doi:10.1089/nat.2019.0796
Takahashi, H., Shimizu, T. and Okano, T. (2018). Engineered human contractile myofiber sheets as a platform for studies of skeletal muscle physiology. Sci Rep 8, 13932. doi:10.1038/s41598-018-32163-1
Tessier, Y., Achanzar, W., Mihalcik, L. et al. (2021). Outcomes of the European federation of pharmaceutical industries and associations oligonucleotide working group survey on nonclinical practices and regulatory expectations for therapeutic oligonucleotide safety assessment. Nucleic Acid Ther 31, 7-20. doi:10.1089/nat.2020.0892
Thomas, P. and Summers, J. W. (1978). The biliary excretion of circulating asialoglycoproteins in the rat. Biochem Biophys Res Commun 80, 335-339. doi:10.1016/0006-291x(78)90681-2
Tian, Q., Rogness, J., Meng, M. et al. (2017). Quantitative determination of a siRNA (AD00370) in rat plasma using peptide nucleic acid probe and HPLC with fluorescence detection. Bioanalysis 9, 861-872. doi:10.4155/bio-2017-0017
Trujillo-de Santiago, G., Flores-Garza, B. G., Tavares-Negrete, J. A. et al. (2019). The tumor-on-chip: Recent advances in the development of microfluidic systems to recapitulate the physiology of solid tumors. Materials (Basel) 12, 2945. doi:10.3390/ma12182945
Truskey, G. A. (2018). Development and application of human skeletal muscle microphysiological systems. Lab Chip 18, 3061-3073. doi:10.1039/c8lc00553b
Urciuolo, A., Serena, E., Ghua, R. et al. (2020). Engineering a 3D in vitro model of human skeletal muscle at the single fiber scale. PLoS One 15, e0232081. doi:10.1371/journal.pone.0232081
Verheul, R. C., van Deutekom, J. C. and Datson, N. A. (2016). Digital droplet PCR for the absolute quantification of exon skipping induced by antisense oligonucleotides in (pre-)clinical development for Duchenne muscular dystrophy. PLoS One 11, e0162467. doi:10.1371/journal.pone.0162467
Viney, N. J., Guo, S., Tai, L. J. et al. (2021). Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: Preclinical and phase 1 data. ESC Heart Fail 8, 652-661. doi:10.1002/ehf2.13154
Visentin, G. P. and Liu, C. Y. (2007). Drug-induced thrombocytopenia. Hematol Oncol Clin North Am 21, 685-696, vi. doi:10.1016/j.hoc.2007.06.005
Wang, D., Zhang, C., Wang, B. et al. (2019). Optimized CRISPR guide RNA design for two high-fidelity Cas9 variants by deep learning. Nat Commun 10, 4284. doi:10.1038/s41467-019-12281-8
Wray, S. (2020). Modelling neurodegenerative disease using brain organoids. Semin Cell Dev Biol 111, 60-66. doi:10.1016/j.semcdb.2020.05.012
Wright, C. B., Becker, S. M., Low, L. A. et al. (2020). Improved ocular tissue models and eye-on-a-chip technologies will facilitate ophthalmic drug development. J Ocul Pharmacol Ther 36, 25-29. doi:10.1089/jop.2018.0139
Yang, N. J. and Hinner, M. J. (2015). Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol Biol 1266, 29-53. doi:10.1007/978-1-4939-2272-7_3
Yang, Q., Humphreys, S. C., Lade, J. M. et al. (2020). Prolonged cultured human hepatocytes as an in vitro experimental system for the evaluation of potency and duration of activity of RNA therapeutics: Demonstration of prolonged duration of gene silencing effects of a GalNAc-conjugated human hypoxanthine phosphoribosyl transferase (HPRT1) siRNA. Biochem Pharmacol 189, 114374. doi:10.1016/j.bcp.2020.114374
Young, A. T., Rivera, K. R., Erb, P. D. et al. (2019). Monitoring of microphysiological systems: Integrating sensors and real-time data analysis toward autonomous decision-making. ACS Sens 4, 1454-1464. doi:10.1021/acssensors.8b01549
Zapata-Linares, N., Rodriguez, S., Salido, E. et al. (2016). Generation and characterization of human iPSC lines derived from a primary hyperoxaluria type I patient with p.I244T mutation. Stem Cell Res 16, 116-119. doi:10.1016/j.scr.2015.12.014
Zhang, Q. J., Li, J. J., Lin, X. et al. (2017). Modeling the phenotype of spinal muscular atrophy by the direct conversion of human fibroblasts to motor neurons. Oncotarget 8, 10945-10953. doi:10.18632/oncotarget.14641
Zhao, A., Pan, Y. and Cai, S. (2020). Patient-specific cells for modeling and decoding amyotrophic lateral sclerosis: Advances and challenges. Stem Cell Rev Rep 16, 482-502. doi:10.1007/s12015-019-09946-8
Zhu, G. and Chen, X. (2018). Aptamer-based targeted therapy. Adv Drug Deliv Rev 134, 65-78. doi:10.1016/j.addr.2018.08.005