Dear readers,
The staff at ALTEX and the board of ALTEX Edition are deeply saddened to say a final goodbye to former editor-in-chief of ALTEX, our esteemed colleague and friend Franz P. Gruber. Franz’ life achievements were celebrated earlier this year in an editorial published on occasion of his 80th birthday (ALTEX 40, 1). With his passing, the 3Rs field has lost one of its most dedicated pioneers.
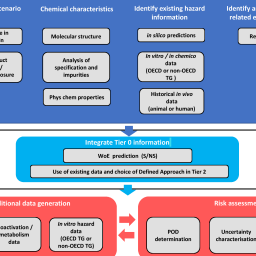
The purpose of REACH, the European Chemicals Regulation, is to protect human health and the environment from risks posed by chemicals. But REACH’s aims also include promoting alternatives to animal experimentation, and it demands that testing on vertebrate animals for the purposes of the Regulation should be undertaken only as a “last resort”. Based on publicly available data, Jean Knight and colleagues calculate that testing for the three systemic toxicity endpoints, which are the most animal intensive, consumed 2.9 million animals and further tests requiring 1.3 million animals were underway up to December 2022. This total of 4.2 million animals already by far exceeds initial estimates.
However, a recent amendment to the Regulation calls for additional animal tests, and a further amendment is under discussion, which will likely also require extensive new tests. Costanza Rovida et al. provide Food for Thought … by estimating the number of animals already consumed for the other endpoints and discussing the challenges posed by the demands of the recent and planned amendments as well as estimates of the number of animals that may be needed if these are to be fulfilled with animal tests. The “exploding head” emoji used in their figure says it all: It is time for the successes of new approach methodologies to be fully implemented in order to minimize the use of animals while effectively protecting human health and the environment.
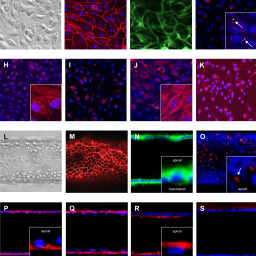
Pedro Caetano-Pinto et al. demonstrate that active drug secretion, which did not occur in their conventional renal cell culture model, can be studied in their microphysiological human kidney model. Renal clearance of drugs in humans was accurately predicted using this in vitro model together with pharmacological modeling tools. The model could help to reduce the use of animals in preclinical drug development.
The GARD™skin assay is the first OECD guideline test that uses genomic data as its endpoint. Andy Forreryd et al. demonstrate the ability of this non-animal assay to detect the skin sensitization potential of metals, supporting that metals can be included in the assay’s applicability domain.
Defined approaches have been developed to interpret results obtained by combinations of different new approach methodologies. Nicola Gilmour et al. compare the outcome of seven defined approaches for skin sensitization testing for two hypothetical use scenarios of a case study cosmetic ingredient and analyze why not all reach the same final result. The study can help to refine the safety assessment of cosmetic ingredients without the use of animals.
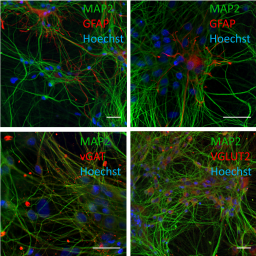
Kristina Bartmann and colleagues describe the development of a human neural network formation and function assay based on the cultivation of human induced pluripotent stem cell (iPSC)-derived excitatory and inhibitory neurons as well as primary human astroglia. The cells grow into a network and generate spontaneous electrical activity patterns that can be detected by microelectrode arrays. Comparison to a similar assay with rat cells showed differences in the two species’ sensitivity to the effects of a variety of pesticides. The new assay can complement the developmental neurotoxicity in vitro testing battery (DNT IVB).
While physiologically based models for predicting the absorption of drugs from the gut have been developed, it is generally assumed that chemicals taken up from the environment will be fully absorbed. Hsing-Chieh Lin and Weihsueh Chiu present a model that uses data from in vitro permeability studies and information on the permeability of different gut segments to predict the absorption of environmental chemicals in the human intestine for risk assessment. They point out that the simple in vitro models used for this purpose to date need to be improved to enable better predictivity.
In their review, which is part of the Special Issue on Microphysiological Systems in Drug Discovery and Safety (doi:10.14573/altex22S1), Onyi Irrechukwu and colleagues consider how microphysiological systems can be used to develop more human-relevant disease models in pharmaceutical drug discovery in order to improve efficiency, safety predictions, and translatability. Such models can reduce or replace the use of animals for this purpose in drug development..
Jan Turner and colleagues report on a workshop on incorporating new approach methodologies into regulatory pharmaceutical safety assessment, and Patricia Bishop et al. provide a t4 Workshop Report on phasing out dog use in the evaluation of agrochemicals. Further updates on recent activities can be found in the Meeting Reports and the Corners.
In case you missed the MPS World Summit in Berlin, you can access the Abstract Book at doi:10.58847/ap.2301. ALTEX Proceedings will also be publishing the Abstract Book for WC12 in Niagara Falls, Canada this August on https://proceedings.altex.org/.
Hoping you are inspired by this issue of ALTEX,
Sonja von Aulock
Editor-in-chief
REACH out-numbered! The future of REACH and animal numbers
https://doi.org/10.14573/altex.2307121
4.2 million and counting… The animal toll for REACH systemic toxicity studies
https://doi.org/10.14573/altex.2303201
The GARD™skin assay: Investigation of the applicability domain for metals
https://doi.org/10.14573/altex.2203021
Incorporating new approach methodologies into regulatory nonclinical pharmaceutical safety assessment
https://doi.org/10.14573/altex.2212081
Challenges and opportunities for overcoming dog use in agrochemical evaluation and registration
https://doi.org/10.14573/altex.2302151
Animal research ethics as interaction of research ethics, animal ethics, and (animal protection) law
https://doi.org/10.14573/altex.2301171
Women in Alternatives
https://doi.org/10.14573/altex.2303211