A call for a Human Exposome Project
Main Article Content
Abstract
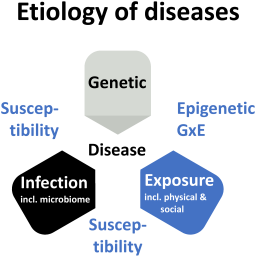
Four decades of the Human Genome Project and its consequences have shown how the entrepreneurial state, through significant investment into science, can drive scientific progress and advance biomedicine. A certain fraction of diseases can now be explained as caused by genetics, and a more significant fraction as impacted by genetics. Besides another fraction caused by pathogens, the third and probably largest impactor is exposure, i.e., the many physicochemical and lifestyle factors. This article makes the case that it is time to start a Human Exposome Project, which systematically explores and catalogs the exposure side of human health and disease.
The envisioned Human Exposome Project needs to be more than a scaled exposomics approach, aiming to assess the totality of relevant exposures through ~omics of human body fluids and forming exposure hypotheses. Exposomics is increasingly complemented by exposure science and biomonitoring to measure exposure, mechanistic understanding, human-relevant microphysiological systems, big data, and artificial intelligence (AI) to mine these data and integrate pieces of evidence. The potential impact of AI on a possible Human Exposome Project is so substantial that we should speak of exposome intelligence (EI) because this allows us to expand our limited current knowledge to the big unknown unknowns of threats to human health.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Articles are distributed under the terms of the Creative Commons Attribution 4.0 International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is appropriately cited (CC-BY). Copyright on any article in ALTEX is retained by the author(s).
Abedini, J., Cook, B., Bell, S. et al. (2021). Application of new approach methodologies: ICE tools to support chemical evaluations. Comput Toxicol 20, 100184. doi:10.1016/j.comtox.2021.100184
Aguilera, O., Fernandez, A. F., Munoz, A. et al. (2010). Epigenetics and environment: A complex relationship. J Appl Physiol 109, 243-251. doi:10.1152/japplphysiol.00068.2010
Alépée, N., Bahinski, A., Daneshian, M. et al. (2014). State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX 31, 441-477. doi:10.14573/altex1406111
Alexander, J. C., Pandit, A., Bao, G. et al. (2011). Monitoring mRNA in living cells in a 3D in vitro model using TAT-peptide linked molecular beacons. Lab Chip 11, 3908-3914. doi:10.1039/c1lc20447e
Andersen, M. E., Clewell, H. J. III, Carmichael, P. L. et al. (2011). Can case study approaches speed implementation of the NRC report: Toxicity testing in the 21st century: A vision and a strategy? ALTEX 28, 175-182. doi:10.14573/altex.2011.3.175
Andersen, M. E., Betts, K., Dragan, Y. et al. (2014). Developing microphysiological systems for use as regulatory tools – Challenges and opportunities. ALTEX 31, 364-367. doi:10.14573/altex.1405151
Andersen, M. E., McMullen, P. D., Phillips, M. B. et al. (2019). Developing context appropriate toxicity testing approaches using new alternative methods (NAMs). ALTEX 36, 523-534. doi:10.14573/altex.1906261
Ankley, G. T., Bennett, R. S., Erickson, R. J. et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29, 730-741. doi:10.1002/etc.34
Aschner, M., Ceccatelli, S., Daneshian, M. et al. (2017). Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: Example lists and criteria for their selection and use. ALTEX 34, 49-74. doi:10.14573/altex.1604201
Aschner, M., Paoliello, M. M. B., Tsatsakis, A. et al. (2021). Social injustice in environmental health: A call for fortitude. Environ Res 194, 110675. doi:10.1016/j.envres.2020.110675
Astashkina, A. and Grainger D.W. (2014). Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69-70, 1-18. doi:10.1016/j.addr.2014.02.008
Ball, N., Cronin, M. T. D., Shen, J. et al. (2016). Toward good read-across practice (GRAP) guidance. ALTEX 33, 149-166. doi:10.14573/altex.1601251
Bal-Price A., Hogberg, H. T., Crofton, K. M. et al. (2018). Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX 35, 306-352. doi:10.14573/altex.1712081
Basketter, D. A., Clewell, H., Kimber, I. et al. (2012). A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing. ALTEX 29, 3-89. doi:10.14573/altex.2012.1.003
Baxi, V., Edwards, R., Montalto, M. et al. (2022). Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod Pathol 35, 23-32. doi:10.1038/s41379-021-00919-2
Beger, R. D., Dunn, W. B., Bandukwala, A. et al. (2019). Towards quality assurance and quality control in untargeted metabolomics studies. Metabolomics 15, 4. doi:10.1007/s11306-018-1460-7
Beilmann, M., Boonen, H., Czich, A. et al. (2019). Optimizing drug discovery by investigative toxicology: Current and future trends. ALTEX 36, 3-17. doi:10.14573/altex.1808181
Beinart, R., Zhang, Y., Lima, J. A. et al. (2014). The QT interval is associated with incident cardiovascular events: The MESA study. J Am Coll Cardiol 64, 2111-2119. doi:10.1016/j.jacc.2014.08.039
Bhattacharya, S., Zhang, Q., Carmichael, P. L. et al. (2011). Toxicity testing in the 21 century: Defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS One 6, e20887. doi:10.1371/journal.pone.0020887
Blakey, D., Galloway, S. M., Kirkland, D. J. et al. (2008). Regulatory aspects of genotoxicity testing: From hazard identification to risk assessment. Mutat Res 657, 84-90. doi:10.1016/j.mrgentox.2008.09.004
Blaauboer, B. J., Boekelheide, K., Clewell, H. J. et al. (2012). The use of biomarkers of toxicity for integrating in vitro hazard estimates into risk assessment for humans. ALTEX 29, 411-425. doi:10.14573/altex.2012.4.411
Boekelheide, K. and Campion, S. N. (2010). Toxicity testing in the 21st century: Using the new toxicity testing paradigm to create a taxonomy of adverse effects. Toxicol Sci 114, 20-24. doi:10.1093/toxsci/kfp307
Bottini, A. A., Amcoff, P. and Hartung, T. (2007). Food for thought… on globalization of alternative methods. ALTEX 24, 255-261. doi:10.14573/altex.2007.4.255
Bottini, A. A. and Hartung, T. (2009). Food for thought ... on the economics of animal testing. ALTEX 26, 3-16. doi:10.14573/altex.2009.1.3
Bouhifd, M., Andersen, M. E., Baghdikian, C. et al. (2015a). The human toxome project. ALTEX 32, 112-124. doi:10.14573/altex.1502091
Bouhifd, M., Beger, R., Flynn, T. et al. (2015b). Quality assurance of metabolomics. ALTEX 32, 319-326. doi:10.14573/altex.1509161
Briggs, K., Bosc, N., Camara, T. et al. (2021). Guidelines for FAIR sharing of preclinical safety and off-target pharmacology data. ALTEX 38, 187-197. doi:10.14573/altex.2011181
Browne P., Delrue, N. and Gourmelon, A. (2019). Regulatory use and acceptance of alternative methods for chemical hazard identification. Curr Op Toxicol 15, 18-25. doi:10.1016/j.cotox.2019.02.003
Bunge, J. A., and Judson, D. H. (2005). Data Mining. In K. Kempf-Leonard (ed.), Encyclopedia of Social Measurement (617-624). Elsevier. doi:10.1016/B0-12-369398-5/00159-6
Busquet, F. and Hartung, T. (2017). The need for strategic development of safety sciences. ALTEX 34, 3-21. doi:10.14573/altex.1701031
Busquet, F., Kleensang, A., Rovida, C. et al. (2020). New European Union statistics on laboratory animal use – What really counts! ALTEX 37, 167-186. doi:10.14573/altex.2003241
Callaway, E. (2022). AlphaFold’s new rival? Meta AI predicts shape of 600 million proteins. Nature 611, 211-212. doi:10.1038/d41586-022-03539-1
Caloni, F., De Angelis, I. and Hartung, T. (2022). Replacement of animal testing by integrated approaches to testing and assessment (IATA): A call for in vivitrosi. Arch Toxicol 96, 1935-1950. doi:10.1007/s00204-022-03299-x
Carmichael, P. L., Baltazar, M. T., Cable, S. et al. (2022). Ready for regulatory use: NAMs and NGRA for chemical safety assurance. ALTEX 39, 359-366. doi:10.14573/altex.2204281
Cassotta, M., Geerts, H., Harbom, L. et al. (2022). The future of Parkinson’s disease research: A new paradigm of human-specific investigation is necessary… and possible. ALTEX 39, 694-709. doi:10.14573/altex.2203161
Chesnut, M., Yamada, T., Adams, T. et al. (2018). Regulatory acceptance of read-across: Report from an international satellite meeting at the 56th annual meeting of the society of toxicology. ALTEX 35, 413-419. doi:10.14573/altex.1805081
Coecke, S., Balls, M., Bowe, G. et al. (2005). Guidance on good cell culture practice – A report of the second ECVAM task force on good cell culture practice. Altern Lab Anim 33, 261-287. doi:10.1177/026119290503300313
Cooper-Hannan, R., Harbell, J. W., Coecke, S. et al. (1999). The principles of good laboratory practice: Application to in vitro toxicology studies – The report and recommendations of ECVAM workshop 37. Altern Lab Anim 27, 539-577. doi:10.1177/026119299902700410
Corvi, R., Albertini, S., Hartung, T. et al. (2008). ECVAM retrospective validation of in vitro micronucleus test (MNT). Mutagenesis 23, 271-283. doi:10.1093/mutage/gen010
Daneshian, M., Akbarsha, M. A., Blaauboer, B. et al. (2011). A framework program for the teaching of alternative methods (replacement, reduction, refinement) to animal experimentation. ALTEX 28, 341-352. doi:10.14573/altex.2011.4.341
Daneshian, M., Botana, L. M., Dechraoui Bottein, M. Y. et al. (2013). A roadmap for hazard monitoring and risk assessment of marine biotoxins on the basis of chemical and biological test systems. ALTEX 30, 487-545. doi:10.14573/altex.2013.4.487
Daneshian, M., Busquet, F., Hartung, T. et al. (2015). Animal use for science in Europe. ALTEX 32, 261-274. doi:10.14573/altex.1509081
Dawson, H. (2022). Digital pathology – Rising to the challenge. Front Med 9, 888896. doi:10.3389/fmed.2022.888896
de Souza Santos, R., Frank, A. P., Palmer, B. F. et al. (2018). Sex and media: Considerations for cell culture studies. ALTEX 35, 435-440. doi:10.14573/altex.1806151
de Vries, R. B. M., Angrish, M., Browne, P et al. (2021). Applying evidence-based methods to the development and use of adverse outcome pathways. ALTEX 38, 336-347. doi:10.14573/altex.2101211
Desai, H. V., Voruganti, I. S., Jayasuriya, C. et al. (2014). Live-cell, temporal gene expression analysis of osteogenic differentiation in adipose-derived stem cells. Tissue Eng Part A 20, 899-907. doi:10.1089/ten.tea.2013.0761
Dolinoy, D.C., Huang, D. and Jirtle, R. L. (2007). Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A 104, 13056-13061. doi:10.1073/pnas.0703739104
Dye, B. R., Hill, D. R. Ferguson, M. A. et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, e05098. doi:10.7554/eLife.05098
EFSA – European Food Safety Authority and EBTC – Evidence-Based Toxicology Collaboration (2018). EFSA scientific colloquium 23: Evidence integration in risk assessment: The science of combining apples and oranges. EFSA Supporting Publications 15, 1396E. doi:10.2903/sp.efsa.2018.EN-1396
EFSA, Tarazona, J., Kass, G. et al. (2022). New approach methodologies. EFSA Supporting Publications 19, e200502. doi:10.2903/sp.efsa.2022.e200502
Eisenman, S. F. (2016). Criticizing animal experimentation, at my peril. ALTEX 33, 3-12. doi:10.14573/altex.1509282
Ellison, C. A., Api, A. M., Becker, R. A. et al. (2020). Internal threshold of toxicological concern (iTTC): Where we are today and what is possible in the near future. Front Toxicol 2, 621541. doi:10.3389/ftox.2020.621541
Engle, S. J. and Vincent, F. (2014). Small molecule screening in human induced pluripotent stem cell-derived terminal cell types. J Biol Chem 289, 4562-4570. doi:10.1074/jbc.r113.529156
Escher, B. I., Hackermüller, J., Polte, T. et al. (2017). From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 99, 97-106. doi:10.1016/j.envint.2016.11.029
Escher, S. E., Partosch, F., Konzok, S. et al. (2022). Development of a roadmap for action on new approach methodologies in risk assessment. EFSA Supporting Publication 19, 7341E. doi:10.2903/sp.efsa.2022.EN-7341
Evans, A. M., O’Donovan, C., Playdon, M. et al. (2020). Dissemination and analysis of the quality assurance (QA) and quality control (QC) practices of LC-MS based untargeted metabolomics practitioners. Metabolomics 16, 113. doi:10.1007/s11306-020-01728-5
Farhat, N., Tsaioun, K., Saunders-Hastings, P. et al. (2022). Systematic review in evidence-based risk assessment. ALTEX 39, 463-479. doi:10.14573/altex.2004111
Ferrario, D. and Rabbit, R. R. (2012). Analysis of the proposed EU regulation concerning biocide products and its opportunities for alternative approaches and a toxicology for the 21st century. ALTEX 29, 157-172. doi:10.14573/altex.2012.2.157
Ferrario, D., Brustio, R. and Hartung, T. (2014). Glossary of reference terms for alternative test methods and their validation. ALTEX 31, 319-335. doi:10.14573/altex.1403311
Fowle, J. J. R. III, Curren, R. D., Hartung, T. et al. (2017). Twenty-first century in vitro toxicology testing methods and the assessment of e-cigarettes. Appl In Vitro Toxicol 3, 3-9. doi:10.1089/aivt.2017.29011.rtl
Fu, Y., Luechtefeld, T., Karmaus, A. et al. (2023). Chapter 39 – The use of artificial intelligence and big data for the safety evaluation of US food-relevant chemicals. In M. E. Knowles, L. E. Anelich, A. R. Boobis et al., Present Knowledge in Food Safety (575-589). Academic Press. doi:10.1016/B978-0-12-819470-6.00061-5
Godoy, P., Hewitt, N. J., Albrecht, U. et al. (2013). Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87, 1315-1530. doi:10.1007/s00204-013-1078-5
Gordon, S., Daneshian, M., Bouwstra, J. et al. (2015). Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. ALTEX 32, 327-378. doi:10.14573/altex.1510051
Grandjean, P. and Bellanger, M. (2017). Calculation of the disease burden associated with environmental chemical exposures: Application of toxicological information in health economic estimation. Environ Health 16, 123. doi:10.1186/s12940-017-0340-3
Grønbæk, M. (2009). The positive and negative health effects of alcohol- and the public health implications. J Intern Med 265, 407-420. doi:10.1111/j.1365-2796.2009.02082.x
Gstraunthaler, G. and Hartung, T. (1999). Bologna declaration toward good cell culture practice. Altern Lab Anim 27, 206.
Guillen, J. (2012). FELASA guidelines and recommendations. J Am Assoc Lab Anim Sci 51, 311-321.
Hardy, B., Apic, G., Carthew, P. et al. (2012). Food for thought ... a toxicology ontology roadmap. ALTEX 29, 129-137. doi:10.14573/altex.2012.2.129
Hartung, T., Balls, M., Bardouille, C. et al. (2002). Good cell culture practice. ECVAM good cell culture practice task force report 1. Altern Lab Anim 30, 407-414. doi:10.1177/026119290203000404
Hartung, T., Bremer, S., Casati, S. et al. (2004). A modular approach to the ECVAM principles on test validity. Altern Lab Anim 32, 467-472. doi:10.1177/026119290403200503
Hartung, T. (2007a). Food for thought ... on validation. ALTEX 24, 67-72. doi:10.14573/altex.2007.2.67
Hartung, T. (2007b). Food for thought ... on cell culture. ALTEX 24, 143-147. doi:10.14573/altex.2007.3.143
Hartung, T. (2008a). Food for thought ... on alternative methods for cosmetics safety testing. ALTEX 25, 147-162. doi:10.14573/altex.2008.3.147
Hartung, T. (2008b). Food for thought ... on animal tests. ALTEX 25, 3-16. doi:10.14573/altex.2008.1.3
Hartung, T. and Leist, M. (2008). Food for thought ... on the evolution of toxicology and the phasing out of animal testing. ALTEX 25, 91-102. doi:10.14573/altex.2008.2.91
Hartung, T. and Koëter, H. (2008). Food for thought ... on food safety testing. ALTEX 25, 259-264. doi:10.14573/altex.2008.4.259
Hartung, T. (2009a). A toxicology for the 21st century: Mapping the road ahead. Tox Sci 109, 18-23. doi:10.1093/toxsci/kfp059
Hartung, T. (2009b). Food for thought ... on evidence-based toxicology. ALTEX 26, 75-82. doi:10.14573/altex.2009.2.75
Hartung, T. and Hoffmann, S. (2009). Food for thought ... on in silico methods in toxicology. ALTEX 26, 155-166. doi:10.14573/altex.2009.3.155
Hartung, T., Blaauboer, B. and Leist, M. (2009). Food for thought ... on education in alternative methods in toxicology. ALTEX 26, 255-263. doi:10.14573/altex.2009.4.255
Hartung, T. (2010a). Evidence based-toxicology – The toolbox of validation for the 21st century? ALTEX 27, 241-251. doi:10.14573/altex.2010.4.253
Hartung, T. (2010b). Food for thought ... on alternative methods for nanoparticle safety testing. ALTEX 27, 87-95. doi:10.14573/altex.2010.2.87
Hartung, T. (2010c). Food for thought ...on alternative methods for chemical safety testing. ALTEX 27, 3-14. doi:10.14573/altex.2010.1.3
Hartung, T. (2010d). Comparative analysis of the revised Directive 2010/63/EU for the protection of laboratory animals with its predecessor 86/609/EEC. ALTEX 27, 285-303. doi:10.14573/altex.2010.4.285
Hartung, T. and McBride, M. (2011). Food for thought… on mapping the human toxome. ALTEX 28, 83-93. doi:10.14573/altex.2011.2.083
Hartung, T., Blaauboer, B. J., Bosgra, S. et al. (2011). An expert consortium review of the EC-commissioned report “Alternative (non-animal) methods for cosmetics testing: Current status and future prospects – 2010”. ALTEX 28, 183-209. doi:10.14573/altex.2011.3.183
Hartung, T. and Zurlo, J. (2012). Alternative approaches for medical countermeasures to biological and chemical terrorism and warfare. ALTEX 29, 251-260. doi:10.14573/altex.2012.3.251
Hartung, T., van Vliet, E., Jaworska, J. et al. (2012). Systems toxicology. ALTEX 29, 119-128. doi:10.14573/altex.2012.2.119
Hartung, T. (2013). Look back in anger – What clinical studies tell us about preclinical work. ALTEX 30, 275-291. doi:10.14573/altex.2013.3.275
Hartung, T. and Corsini, E. (2013). Immunotoxicology: Challenges in the 21st century and in vitro opportunities. ALTEX 30, 411-426. doi:10.14573/altex.2013.4.411
Hartung, T., Luechtefeld, T., Maertens, A. et al. (2013a). Integrated testing strategies for safety assessments. ALTEX 30, 3-18. doi:10.14573/altex.2013.1.003
Hartung, T., Stephens, M. and Hoffmann, S. (2013b). Mechanistic validation. ALTEX 30, 119-130. doi:10.14573/altex.2013.2.119
Hartung, T. (2015). The human whole blood pyrogen test – Lessons learned in twenty years. ALTEX 32, 79-100. doi:10.14573/altex.1503241
Hartung, T. (2016a). Making big sense from big data in toxicology by read-across. ALTEX 33, 83-93. doi:10.14573/altex.1603091
Hartung, T. (2016b). E-Cigarettes and the need and opportunities for alternatives to animal testing. ALTEX 33, 211-224. doi:10.14573/altex.1606291
Hartung, T. (2016c). Can we wait for e-cigarette trials? Chemistry World. http://www.rsc.org/chemistryworld/2016/04/electronic-cigarettes-trials-vaping-safety-data
Hartung, T. (2016d). E-cigarettes – The ugly duckling of public health? Sci Am 315, 9.
Hartung, T. (2017a). Thresholds of toxicological concern – Setting a threshold for testing where there is little concern. ALTEX 34, 331-351. doi:10.14573/altex.1707011
Hartung, T. (2017b). Opinion versus evidence for the need to move away from animal testing. ALTEX 34, 193-200. doi:10.14573/altex.1703291
Hartung, T. (2017c). Utility of the adverse outcome pathway concept in drug development. Expert Opin Drug Metab Toxicol 13, 1-3. doi:10.1080/17425255.2017.1246535
Hartung, T. (2017d). Food for thought … the first ten years. ALTEX 34, 187-192. doi:10.14573/altex.1703311
Hartung, T., FitzGerald, R., Jennings, P.et al. (2017). Systems toxicology – Real world applications and opportunities. Chem Res Toxicol 30, 870-882. doi:10.1021/acs.chemrestox.7b00003
Hartung, T. (2018a). Rebooting the generally recognized as safe (GRAS) approach for food additive safety in the US. ALTEX 35, 3-25. doi:10.14573/altex.1712181
Hartung, T. (2018b). Making big sense from big data. Front Big Data 1, 5. doi:10.3389/fdata.2018.00005
Hartung, T. (2018c). Perspectives on in vitro to in vivo extrapolations. J Appl In Vitro Toxicol 4, 305-316. doi:10.1089/aivt.2016.0026
Hartung, T., de Vries, R., Hoffmann, S. et al. (2019). Toward good in vitro reporting standards. ALTEX 36, 3-17. doi:10.14573/altex.1812191
Hartung, T. (2021a). Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood test. ALTEX 38, 3-19. doi:10.14573/altex.2101051
Hartung, T. (2021b). Evidence integration in the era of information flooding – The advent of the comprehensive review. Front Public Health 9, 763828. doi:10.3389/fpubh.2021.763828
Hartung, T. and Tsatsakis, A. M. (2021). The state of the scientific revolution in toxicology. ALTEX 38, 379-386. doi:10.14573/altex.2106101
Hasiwa, N., Bailey, J., Clausing, P. et al. (2011). Critical evaluation of the use of dogs in biomedical research and testing in Europe. ALTEX 28, 326-340. doi:10.14573/altex.2011.4.326
Hasiwa, N., Daneshian, M., Bruegger, P. et al. (2013). Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX 30, 169-208. doi:10.14573/altex.2013.2.169
Heinonen, T. (2015). Better science with human cell-based organ and tissue models. Altern Lab Anim 43, 29-38. doi:10.1177/026119291504300107
Helman, G., Shah, I., Williams, A. J. et al. (2019). Generalized read-across (GenRA): A workflow implemented into the EPA CompTox chemicals dashboard. ALTEX 36, 462-465. doi:10.14573/altex.1811292
Hermann, K., Pistollato, F. and Stephens, M. (2019). Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. ALTEX 36, 343-352. doi:10.14573/altex.1907031
Herzler, M., Marx-Stoelting, P., Pirow, R. et al. (2021). The “EU chemicals strategy for sustainability” questions regulatory toxicology as we know it: Is it all rooted in sound scientific evidence? Arch Toxicol 95, 2589-2601. doi:10.1007/s00204-021-03091-3
Hoffmann, S. and Hartung, T. (2006a). Designing validation studies more efficiently according to the modular approach: Retrospective analysis of the EPISKIN test for skin corrosion. Altern Lab Anim 34, 177-191. doi:10.1177/026119290603400209
Hoffmann, S. and Hartung, T. (2006b). Towards an evidence-based toxicology. Hum Exp Toxicol 25, 497-513. doi:10.1191/0960327106het648oa
Hoffmann, S., Edler, L., Gardner, I. et al. (2008). Points of reference in validation – The report and recommendations of ECVAM workshop. Altern Lab Anim 36, 343-352. doi:10.1177/026119290803600311
Hoffmann, S., de Vries, R. B. M., Stephens, M. L. et al. (2017). A primer on systematic reviews in toxicology. Arch Toxicol 91, 2551-2575. doi:10.1007/s00204-017-1980-3
Hoffmann, S., Margliani, B., Akgün-Ölmez, S. G. et al. (2021). A systematic review to compare chemical hazard predictions of the zebrafish embryo test with mammalian prenatal developmental toxicity. Toxicol Sci 183, 14-35. doi:10.1093/toxsci/kfab072
Hoffmann, S., Whaley, P. and Tsaioun, K. (2022a). How evidence-based methodologies can help identify and reduce uncertainty in chemical risk assessment. ALTEX 39, 175-182. doi:10.14573/altex.2201131
Hoffmann, S., Aiassa, E., Angrish, M. et al. (2022b). Application of evidence-based methods to construct mechanism-driven chemical assessment frameworks. ALTEX 39, 499-518. doi:10.14573/altex.2202141
Jacobs, M. N., Versteegen, R. J., Treasure, C. et al. (2019). Addressing potential ethical issues regarding the supply of human-derived products or reagents in in vitro OECD test guidelines. ALTEX 36, 163-176. doi:10.14573/altex.1901281
Jahn, S. W., Plass, M. and Moinfar, F. (2020). Digital pathology: Advantages, limitations and emerging perspectives. J Clin Med 18, 3697. doi:10.3390/jcm9113697
Jones, P. A. and Baylin, S. B. (2007). The epigenomics of cancer. Cell 128, 683-692. doi:10.1016/j.cell.2007.01.029
Jonker, D. M., Kenna, L. A. Leishman, D. et al. (2005). A pharmacokinetic-pharmacodynamic model for the quantitative prediction of dofetilide clinical QT prolongation from human ether-a-go-go-related gene current inhibition data. Clin Pharmacol Ther 77, 572-582. doi:10.1016/j.clpt.2005.02.004
Juberg, D. R., Borghoff, S. J., Becker, R. A. et al. (2014). Lessons learned, challenges, and opportunities: The U.S. endocrine disruptor screening program. ALTEX 31, 63-78. doi:10.14573/altex.1309171
Kang, I., Smirnova, L., Kuhn, J. H. et al. (2021). COVID-19 – Prime time for microphysiological systems, as illustrated for the brain. ALTEX 38, 535-549. doi:10.14573/altex.2110131
Kerecman Myers, D., Goldberg, A. M., Poth, A. et al. (2017). From in vivo to in vitro: The medical device testing paradigm shift. ALTEX 34, 479-500. doi:10.14573/altex.1608081
Kim, J. A., Han, E., Eun, C. J. et al. (2012). Real-time concurrent monitoring of apoptosis, cytosolic calcium, and mitochondria permeability transition for hypermulticolor high-content screening of drug-induced mitochondrial dysfunction-mediated hepatotoxicity. Toxicol Lett 214, 175-181. doi:10.1016/j.toxlet.2012.08.027
Kirkland, D., Pfuhler, S., Tweats, D. et al. (2007). How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: Report of an ECVAM workshop. Mutat Res 628, 31-55. doi:10.1016/j.mrgentox.2006.11.008
Kleensang, A., Maertens, A., Rosenberg, M. et al. (2014). Pathways of toxicity. ALTEX 31, 53-61. doi:10.14573/altex.1309261
Kleinstreuer, N. C., Hoffmann, S., Alépée, N. et al. (2018). Non-animal methods to predict skin sensitization (II): An assessment of defined approaches *. Crit Rev Toxicol 48, 359-374. doi:10.1080/10408444.2018.1429386
Knight, J., Rovida, C., Kreiling, R. et al. (2021). Continuing animal tests on cosmetic ingredients for REACH in the EU. ALTEX 38, 653-668. doi:10.14573/altex.2104221
Kolle, S. N., Landsiedel, R. and Natsch, A. (2020). Replacing the refinement for skin sensitization testing: Considerations to the implementation of adverse outcome pathway (AOP)-based defined approaches (DA) in OECD guidelines. Regul Toxicol Pharmacol 115, 104713. doi:10.1016/j.yrtph.2020.104713
Kostadinova, R., Boess, F., Applegate, D. et al. (2013). A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol 268, 1-16. doi:10.1016/j.taap.2013.01.012
Krebs, A., Waldmann, T., Wilks, M. F. et al. (2019). Template for the description of cell-based toxicological test methods to allow evaluation and regulatory use of the data. ALTEX 36, 682-699. doi:10.14573/altex.1909271
Krewski, D., Acosta, D. Jr., Andersen, M. et al. (2010). Toxicity testing in the 21st century: A vision and a strategy. J Toxicol Environ Health B Crit Rev 13, 51-138. doi:10.1080/10937404.2010.483176
Krewski, D., Andersen, M. E., Tyshenko, M. G. et al. (2020). Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch Toxicol 94, 1-58. doi:10.1007/s00204-019-02613-4
Krewski, D., Saunders-Hastings, P., Baan, R. A. et al. (2022). Development of an evidence-based risk assessment framework. ALTEX 39, 667-693. doi:10.14573/altex.2004041
Kuegler, P. B., Zimmer, B., Waldmann, T. et al. (2010). Markers of murine embryonic and neural stem cells, neurons and astrocytes: Reference points for developmental neurotoxicity testing. ALTEX 27, 17-42. doi:10.14573/altex.2010.1.16
Lakhani, C. M., Tierney, B. T., Manrai, A. K. et al. (2019). Repurposing large health insurance claims data to estimate genetic and environmental contributions in 560 phenotypes. Nat Genet 51, 327-334. doi:10.1038/s41588-018-0313-7
LeCluyse, E. L., Witek, R. P., Andersen, M. E. et al. (2012). Organotypic liver culture models: Meeting current challenges in toxicity testing. Crit Rev Toxicol 42, 501-548. doi:10.3109/10408444.2012.682115
Leist, M., Kadereit, S. and Schildknecht, S. (2008a). Food for thought ... on the real success of 3R approaches. ALTEX 25, 17-32. doi:10.14573/altex.2008.1.17
Leist, M., Bremer, S., Brundin, P. et al. (2008b). The biological and ethical basis of the use of human embryonic stem cells for in vitro test systems or cell therapy. ALTEX 25, 163-190. doi:10.14573/altex.2008.3.163
Leist, M., Efremova, L. and Karreman, C. (2010). Food for thought ... considerations and guidelines for basic test method descriptions in toxicology. ALTEX 27, 309-317. doi:10.14573/altex.2010.4.309
Leist, M., Lidbury, B. A., Yang, C. et al. (2012). Novel technologies and an overall strategy to allow hazard assessment and risk prediction of chemicals, cosmetics, and drugs with animal-free methods. ALTEX 29, 373-388. doi:10.14573/altex.2012.4.373
Leist, M., Hasiwa, N., Rovida, C. et al. (2014). Consensus report on the future of animal-free systemic toxicity testing. ALTEX 31, 341-356. doi:10.14573/altex.1406091
Leist, M., Ghallab, A., Graepel, R. et al. (2017). Adverse outcome pathways: Opportunities, limitations and open questions. Arch Toxicol 31, 221-229. doi:10.1007/s00204-017-2045-3
Lieber, C. S. (1980). Interaction of ethanol with drug toxicity. Am J Gastroenterol 74, 313-320.
Linkov, I., Massey, O., Keisler, J. et al. (2015). From “weight of evidence” to quantitative data integration using multicriteria decision analysis and Bayesian methods. ALTEX 32, 3-8. doi:10.14573/altex.1412231
Lippa, K. A., Aristizabal-Henao, J. J., Beger, R. D. et al. (2022). Reference materials for MS-based untargeted metabolomics and lipidomics: A review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 18, 24. doi:10.1007/s11306-021-01848-6
Locke, P. A. and Myers, D. B. (2011). A replacement-first approach to toxicity testing is necessary to successfully reauthorize TSCA. ALTEX 28, 266-272. doi:10.14573/altex.2011.4.266
Luechtefeld, T. and Hartung, T. (2017). Computational approaches to chemical hazard assessment. ALTEX 34, 459-478. doi:10.14573/altex.1710141
Luechtefeld, T., Marsh, D., Rowlands, C. et al. (2018). Machine learning of toxicological big data enables read-across structure activity relationships (RASAR) outperforming animal test reproducibility. Toxicol Sci 165, 198-212. doi:10.1093/toxsci/kfy152
Madia, F., Corvi, R., Worth, A. et al. (2020). Making better use of toxicity studies for human health by extrapolating across endpoints. ALTEX 37, 519-531. doi:10.14573/altex.2005061
Maertens, A., Anastas, N., Spencer, P. J. et al. (2014). Green toxicology. ALTEX 31, 243-249. doi:10.14573/altex.1406181
Maertens, A., Luechtefeld, T., Kleensang, A. et al. (2015). MPTP’s pathway of toxicity indicates central role of transcription factor SP1. Arch Toxicol 89, 743-755. doi:10.1007/s00204-015-1509-6
Maertens, A., Bouhifd, M., Zhao, L. et al. (2017). Metabolomic network analysis of estrogen-stimulated MCF-7 cells: A comparison of over-representation analysis, quantitative enrichment analysis and pathway analysis versus metabolite network analysis. Arch Toxicol 91, 217-230. doi:10.1007/s00204-016-1695-x
Maertens, A. and Hartung, T. (2018). Green toxicology – Know early about and avoid toxic product liabilities. Toxicol Sci 161, 285-289. doi:10.1093/toxsci/kfx243
Maertens, A., Golden, E., Luechtefeld, T. H. et al. (2022). Probabilistic risk assessment – The keystone for the future of toxicology. ALTEX 39, 3-29. doi:10.14573/altex.2201081
Manikkam, M., Haque, M. M. Guerrero-Bosagna, C. et al. (2014). Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One 9, e102091. doi:10.1371/journal.pone.0102091
Marx, U., Andersson, T. B., Bahinski, A. et al. (2016). Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 33, 272-321. doi:10.14573/altex.1603161
Marx, U., Akabane, T., Andersson, T. et al. (2020). Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37, 365-394. doi:10.14573/altex.2001241
Mazzucato, M. (2013). The Entrepreneurial State: Debunking Public vs. Private Sector Myths. 1st edition . Anthem Press. doi:10.4337/roke.2015.01.10
McNicol, D. (2016). A Primer of Signal Detection Theory. United Kingdom: Taylor & Francis Group.
Meigs, L., Smirnova, L., Rovida, C. et al. (2018). Animal testing and its alternatives – The most important omics is economics. ALTEX 35, 275-305. doi:10.14573/altex.1807041
Miller, G. W. and Jones, D. P. (2014). Nature of nurture: Refining the definition of the exposome. Toxicol Sci 137, 1-2. doi:10.1093/toxsci/kft251
Miller, G. W. (2020). The Exposome – A New Paradigm for the Environment and Health. 2nd edition. Academic Press. doi:10.1016/C2017-0-00630-4
Modafferi, S., Zhong, X., Kleensang, A. et al. (2021). Gene-environment interactions in developmental neurotoxicity: A case study of synergy between chlorpyrifos and CHD8 knockout in human BrainSpheres. Environ Health Perspect 129, 77001. doi:10.1289/EHP8580
Mondou, M., Maguire, S., Pain, G. et al. (2021). Envisioning an international validation process for new approach methodologies in chemical hazard and risk assessment. Environ Adv 4, 100061. doi:10.1016/j.envadv.2021.100061
Muratov, E. N., Bajorath, J., Sheridan, R. P. et al. (2020). QSAR without borders. Chem Soc Rev 49, 3525-3564. doi:10.1039/d0cs00098a
Nantasenamat, C. (2020). Best practices for constructing reproducible QSAR models. In K. Roy (ed.), Ecotoxicological QSARs. Methods in Pharmacology and Toxicology. New York, NY, USA: Humana. doi:10.1007/978-1-0716-0150-1_3
Nelson, P., Urs, N. V. and Kasicheyanula, T. R. (2022). Progress in natural language processing and language understanding. In M. V. Albert, L. Lin, M. J. Spector et al. (eds), Bridging Human Intelligence and Artificial Intelligence. Educational Communications and Technology: Issues and Innovations. Cham, Switzerland: Springer. doi:10.1007/978-3-030-84729-6_6
Nielsen, J. B., Graff, C., Rasmussen, P. V. et al. (2014). Risk prediction of cardiovascular death based on the QTc interval: Evaluating age and gender differences in a large primary care population. Eur Heart J 35, 1335-1344. doi:10.1093/eurheartj/ehu081
NRC –National Research Council (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. New York, USA: The National Academies Press.
O’Brien, P. J. (2014). High-content analysis in toxicology: Screening substances for human toxicity potential, elucidating subcellular mechanisms and in vivo use as translational safety biomarkers. Basic Clin Pharmacol Toxicol 115, 4-17. doi:10.1111/bcpt.12227
Pamies, D. and Hartung, T. (2017). 21st century cell culture for 21st century toxicology. Chem Res Toxicol 30, 43-52. doi:10.1021/acs.chemrestox.6b00269
Pamies, D., Bal-Price, A., Simeonov, A. et al. (2017). Good cell culture practice for stem cells and stem-cell-derived models. ALTEX 34, 95-132. doi:10.14573/altex.1607121
Pamies, D., Bal-Price, A., Chesné, C. et al. (2018). Advanced good cell culture practice for human primary, stem cellderived and organoid models as well as microphysiological systems. ALTEX 35, 353-378. doi:10.14573/altex.1710081
Pamies, D., Leist, M., Coecke, S. et al. (2020). Good cell and tissue culture practice 2.0 (GCCP 2.0) – Draft for stakeholder discussion and call for action. ALTEX 37, 490-492. doi:10.14573/altex.2007091
Pamies, D., Leist, M., Coecke, S. et al. (2022). Guidance document on good cell and tissue culture practice 2.0 (GCCP 2.0). ALTEX 39, 30-70. doi:10.14573/altex.2111011
Paparella, M., Daneshian, M., Hornek-Gausterer, R. et al. (2013). Uncertainty of testing methods – What do we (want to) know? ALTEX 30, 131-144. doi:10.14573/altex.2013.2.131
Parks Saldutti, L., Beyer, B. K., Breslin, W. et al. (2013). In vitro testicular toxicity models: Opportunities for advancement via biomedical engineering techniques. ALTEX 30, 353-377. doi:10.14573/altex.2013.3.353
Partosch, F., Mielke, H., Stahlmann, R. et al. (2015). Internal threshold of toxicological concern values: Enabling route-to-route extrapolation. Arch Toxicol 89, 941-948. doi:10.1007/s00204-014-1287-6
Patlewicz, G., Ball, N., Becker, R. A. et al. (2014). Read-across approaches – Misconceptions, promises and challenges ahead. ALTEX 31, 387-396. doi:10.14573/altex.1410071
Pendse, S. N., Maertens, A., Rosenberg, M. et al. (2017). Information-dependent enrichment analysis reveals time-dependent transcriptional regulation of the estrogen pathway of toxicity. Arch Toxicol 91, 1749-1762. doi:10.1007/s00204-016-1824-6
Persson, M., Loye, A. F., Jacquet, M. et al. (2014). High-content analysis/screening for predictive toxicology: Application to hepatotoxicity and genotoxicity. Basic Clin Pharmacol Toxicol 115, 18-23. doi:10.1111/bcpt.12200
Piir, G., Kahn, I., García-Sosa, A. T. et al. (2018). Best practices for QSAR model reporting: Physical and chemical properties, ecotoxicity, environmental fate, human health, and toxicokinetics endpoints. Environ Health Perspect 126, 126001. doi:10.1289/EHP3264
Price, E. J., Vitale, C. M., Miller, G. W. et al. (2022). Merging the exposome into an integrated framework for “omics” sciences. iScience 25, 103976. doi:10.1016/j.isci.2022.103976
Prüss-Ustün, A., Vickers, C., Haefliger, P. et al. (2011). Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ Health 10, 9. doi:10.1186/1476-069X-10-9
Ramirez, T., Daneshian, M., Kamp, H. et al. (2013). Metabolomics in toxicology and preclinical research. ALTEX 30, 209-225. doi:10.14573/altex.2013.2.209
Rappaport, S. M. and Smith, M. T. (2010). Environment and disease risks. Science 330, 460-461. doi:10.1126/science.1192603
Rossini, G. P. and Hartung, T. (2012). Food for thought … towards tailored assays for cell-based approaches to toxicity testing. ALTEX 29, 359-372. doi:10.14573/altex.2012.4.359
Roth, A. and MPS-WS Berlin (2019). Human microphysiological systems for drug development. Science 373, 1304-1306. doi:10.1126/science.abc3734
Rothen-Rutishauser, B., Blank, F., Muhlfeld, C. et al. (2008). In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol 4, 1075-1089. doi:10.1517/17425255.4.8.1075
Rovida, C. and Hartung, T. (2009). Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals. ALTEX 26, 187-208. doi:10.14573/altex.2009.3.187
Rovida, C. (2010). Food for thought ... why no new in vitro tests will be done for REACH by registrants. ALTEX 27, 175-183. doi:10.14573/altex.2010.3.175
Rovida, C., Longo, F. and Rabbit, R. R. (2011). How are reproductive toxicity and developmental toxicity addressed in REACH dossiers? ALTEX 28, 273-294. doi:10.14573/altex.2011.4.273
Rovida, C., Alépée, N., Api, A. M. et al. (2015a). Integrated testing strategies (ITS) for safety assessment. ALTEX 32, 171-181. doi:10.14573/altex.1411011
Rovida, C., Asakura, S., Daneshian, M. et al. (2015b). Toxicity testing in the 21st century beyond environmental chemicals. ALTEX 32, 171-181. doi:10.14573/altex.1506201
Rovida, C., Barton-Maclaren, T., Benfenati, E. et al. (2020). Internationalisation of read-across as a validated new approach 2 method (NAM) for regulatory toxicology. ALTEX 37, 579-606. doi:10.14573/altex.1912181
Samuel, G. O., Hoffmann, S., Wright, R. et al. (2016). Guidance on assessing the methodological and reporting quality of toxicologically relevant studies: A scoping review. Environ Int 92-93, 630-646. doi:10.1016/j.envint.2016.03.010
Sayette, M. A. (2017). The effects of alcohol on emotion in social drinkers. Behav Res Ther 88, 76-89. doi:10.1016/j.brat.2016.06.005
Schmidt, C. W. (2015). Diversity outbred: A new generation of mouse model. Environ Health Perspect 123, A64-67. doi:10.1289/ehp.123-A64
Silbergeld, E. K., Contreras, E. Q., Hartung, T. et al. (2011). Nanotoxicology: “The end of the beginning” – Signs on the roadmap to a strategy for assuring the safe application and use of nanomaterials. ALTEX 28, 236-241. doi:10.14573/altex.2011.3.236
Sillé, F. C. M., Karakitsios, S., Kleensang, A. et al. (2020). The exposome – A new approach for risk assessment. ALTEX 37, 3-23. doi:10.14573/altex.2001051
Skinner, M. K. (2014). Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol 398, 4-12. doi:10.1016/j.mce.2014.07.019
Smirnova, L., Hogberg, H. T., Leist, M. et al. (2014). Developmental neurotoxicity – Challenges in the 21st century and in vitro opportunities. ALTEX 31, 129-156. doi:10.14573/altex.1403271
Smirnova, L., Harris, G., Leist, M. et al. (2015). Cellular resilience. ALTEX 32, 247-260. doi:10.14573/altex.1509271
Smirnova, L., Kleinstreuer, N., Corvi, R. et al. (2018). 3S – Systematic, systemic, and systems biology and toxicology. ALTEX 35,139-162. doi:10.14573/altex.1804051
Spielmann, H. (2019). Progress in eliminating one-year dog studies for the safety assessment of pesticides. In H. Kojima, T. Seidle, and H. Spielmann (eds), Alternatives to Animal Testing. Singapore: Springer. doi:10.1007/978-981-13-2447-5_6
Steger-Hartmann T., Kreuchwig, A., Vaas, L. et al. (2020). Introducing the concept of virtual control groups into preclinical toxicology animal testing. ALTEX 37, 343-349. doi:10.14573/altex.2001311
Stephens, M. L., Andersen, M., Becker, R. A. et al. (2013). Evidence-based toxicology for the 21st century: Opportunities and challenges. ALTEX 30, 74-104. doi:10.14573/altex.2013.1.074
Stephens, M. L., Betts, K., Beck, N. B. et al. (2016). The emergence of systematic review in toxicology. Toxicol Sci 152, 10-16. doi:10.1093/toxsci/kfw059
Stephens, M., Akgün-Ölmez Gül, S., Hoffmann, S. et al. (2019). Adaptation of the systematic review framework to the assessment of toxicological test methods: Early lessons learned using the zebrafish embryotoxicity test as the test case. Toxicol Sci 171, 56-68. doi:10.1093/toxsci/kfz128
Stucki, A. O., Barton-Maclaren, T. S., Bhuller, Y. et al. (2022). Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front Toxicol 4, 964553. doi:10.3389/ftox.2022.964553
Taylor, K., Stengel, W., Casalegno, C. et al. (2014). Experiences of the REACH testing proposals system to reduce animal testing. ALTEX 31, 107-128. doi:10.14573/altex.1311151
Thomas, R. S., Paules, R. S., Simeonov, A. et al. (2018). The US Federal Tox21 program: A strategic and operational plan for continued leadership. ALTEX 35, 163-168. doi:10.14573/altex.1803011
Threadgill, D. W. and Churchill, G. A. (2012). Ten years of the collaborative cross. Genetics 190, 291-294. doi:10.1534/genetics.111.138032
Ting, A. H., McGarvey, K. M. and Baylin, S. B. (2006). The cancer epigenome – Components and functional correlates. Genes Dev 20, 3215-3231. doi:10.1101/gad.1464906
Tollefsen, K. E., Scholz, S., Cronin, M. T. et al. (2014). Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA). Regul Toxicol Pharmacol 70, 629-640. doi:10.1016/j.yrtph.2014.09.009
Tolosa, L., Pinto, S. and Donato, M. T. (2012). Development of a multiparametric cell-based protocol to screen and classify the hepatotoxicity potential of drugs. Toxicol Sci 127, 187-198. doi:10.1093/toxsci/kfs083
Tolosa, L., Gomez-Lechon, M. J. and Donato, M. T. (2015). High-content screening technology for studying drug-induced hepatotoxicity in cell models. Arch Toxicol 89, 1007-1022. doi:10.1007/s00204-015-1503-z
Tralau, T. and Luch, A. (2015). Moving from rats to cellular omics in regulatory toxicology: Great challenge toward sustainability or “up-shit-creek without a paddle”? Arch Toxicol 89, 819-821. doi:10.1007/s00204-015-1511-z
Tralau, T., Oelgeschläger, M., Gürtler, R. et al. (2015). Regulatory toxicology in the twenty-first century: Challenges, perspectives and possible solutions. Arch Toxicol 89, 823-850. doi:10.1007/s00204-015-1510-0
Trask, O. J., Jr., Moore, A. and LeCluyse, E. L. (2014). A micropatterned hepatocyte coculture model for assessment of liver toxicity using high-content imaging analysis. Assay Drug Dev Technol 12, 16-27. doi:10.1089/adt.2013.525
Tropsha, A. (2010). Best practices for QSAR model development, validation, and exploitation. Mol Inform 29, 476-488. doi:10.1002/minf.201000061
Tsaioun, K., Blaauboer, B. J. and Hartung, T. (2016). Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods. ALTEX 33, 343-358. doi:10.14573/altex.1610101
van der Zalm, A. J., Barroso, J., Browne, P. et al. (2022). A framework for establishing scientific confidence in new approach methodologies. Arch Toxicol 96, 2865-2879. doi:10.1007/s00204-022-03365-4
van Vliet, E., Daneshian, M., Beilmann, M. et al. (2014). Current approaches and future role of high content imaging in safety sciences and drug discovery. ALTEX 31, 479-493. doi:10.14573/altex.1405271
Vinken, M., Benfenati, E., Busquet, F. et al. (2021). Safer chemicals using less animals: Kick-off of the European ONTOX project. Toxicology 458, 152846. doi:10.1016/j.tox.2021.152846
Virchow, R. and Chance, F. (1860). Cellular Pathology, as Based Upon Physiological and Pathological Histology. Twenty Lectures Delivered in the Pathological Institute of Berlin During the Months of February, March and April, 1858. New York, USA: R. M. De Witt. doi:10.5962/bhl.title.110759
von Aulock, S., Busquet, F., Locke, P. et al. (2022). Engagement of scientists with the public and policymakers to promote alternative methods. ALTEX 39, 543-559. doi:10.14573/altex.2209261
Whaley, P., Blaauboer, B. J., Brozek, J. et al. (2021). Improving the quality of toxicology and environmental health systematic reviews: What journal editors can do. ALTEX 38, 513-522. doi:10.14573/altex.2106111
Wilcox, N. and Goldberg, A. (2011). Food for thought ... on validation. A puzzle or a mystery: An approach founded on new science. ALTEX 28, 3-8. doi:10.14573/altex.2011.1.003
Wild, C. P. (2019). The global cancer burden: Necessity is the mother of prevention. Nat Rev Cancer 19, 123-124. doi:10.1038/s41568-019-0110-3
Wink, S., Hiemstra, S., Huppelschoten, S. et al. (2014). Quantitative high content imaging of cellular adaptive stress response pathways in toxicity for chemical safety assessment. Chem Res Toxicol 27, 338-355. doi:10.1021/tx4004038
Zhang, P., Carlsten, C., Chaleckis, R. et al. (2021). Defining the scope of exposome studies and research needs from a multidisciplinary perspective. Environ Sci Technol Lett 12, 839-852. doi:10.1021/acs.estlett.1c00648
Zhang, Y., Post, W.S., Dalal, D. et al. (2011). QT-interval duration and mortality rate: Results from the third national health and nutrition examination survey. Arch Intern Med 171, 1727-1733. doi:10.1001/archinternmed.2011.433
Zhu, H., Zhang, J., Kim, M. T. et al. (2014). Big data in chemical toxicity research: The use of high-throughput screening assays to identify potential toxicants. Chem Res Toxicol 27, 1643-1651. doi:10.1021/tx500145h
Zhu, H., Bouhifd, M., Kleinstreuer, N. et al. (2016). Supporting read-across using biological data. ALTEX 33, 167-182. doi:10.14573/altex.1601252
Zurlo, J., Bayne, K. A., Brown, D. C. et al. (2011). Critical evaluation of the use of dogs in biomedical research and testing in Europe. ALTEX 28, 355-359. doi:10.14573/altex.2011.4.355
Zurlo, J. and Hutchinson, E. (2014). Refinement. ALTEX 31, 4-10. doi:10.14573/altex.1312191